Key points
- PrEP is the use of antiretroviral medication to prevent HIV.
- Inform all sexually active adult and adolescent patients about PrEP.
- Prescribe PrEP to anyone who asks for it, including sexually active people who do not report HIV risk factors.
- Offer PrEP medication and methods of administration to meet patients' needs.

Recommendations
PrEP (pre-exposure prophylaxis) is the use of antiretroviral medication to prevent HIV. PrEP is for people without HIV who may be exposed to HIV through sex or injection drug use.
Consider PrEP as part of a comprehensive HIV prevention plan that includes discussing how to take PrEP as prescribed, proper condom use, screening for other sexually transmitted infections (STIs), and other risk-reduction methods.
Any licensed prescriber can prescribe PrEP. You do not have to specialize in infectious diseases or HIV medicine to prescribe PrEP.
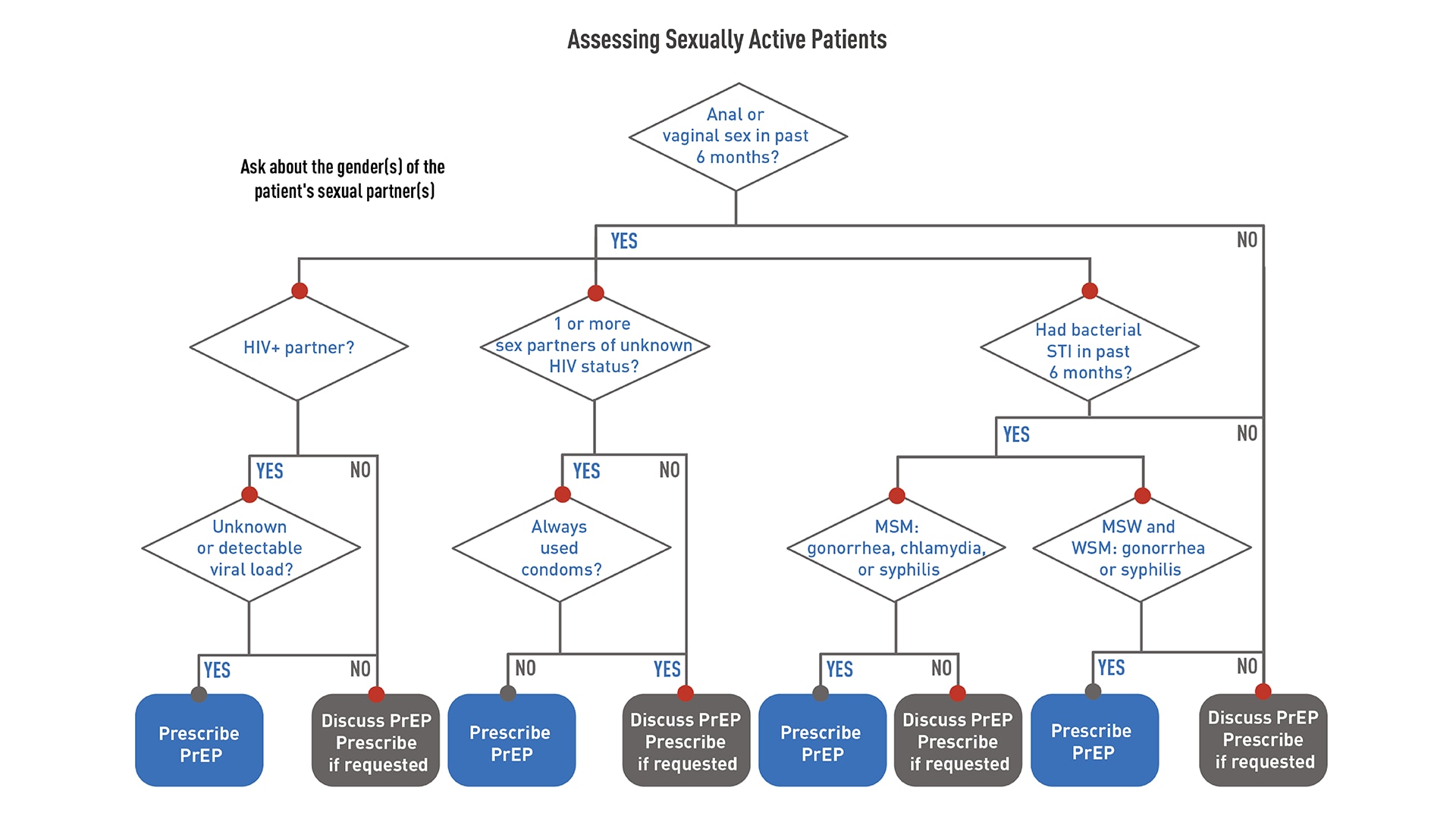
Tell all your sexually active patients without HIV about PrEP. Talking to your patients about PrEP will increase the number of people who know about PrEP. It may also help patients overcome embarrassment or stigma that could prevent them from disclosing their HIV risk factors.123456 Prescribe PrEP to anyone who asks for it, including sexually active adults and adolescents who do not report HIV risk factors.
Have questions about PrEP or need prescribing advice?
Whether or not a patient asks for PrEP, it is important to take a sexual and substance use history. This information is essential to understanding each patient's chances of getting HIV, if PrEP might be right for them, and what other risk-reduction services they might need.
Use the following flowcharts to assess patients before offering PrEP.
Offering PrEP to sexually active adults and adolescents
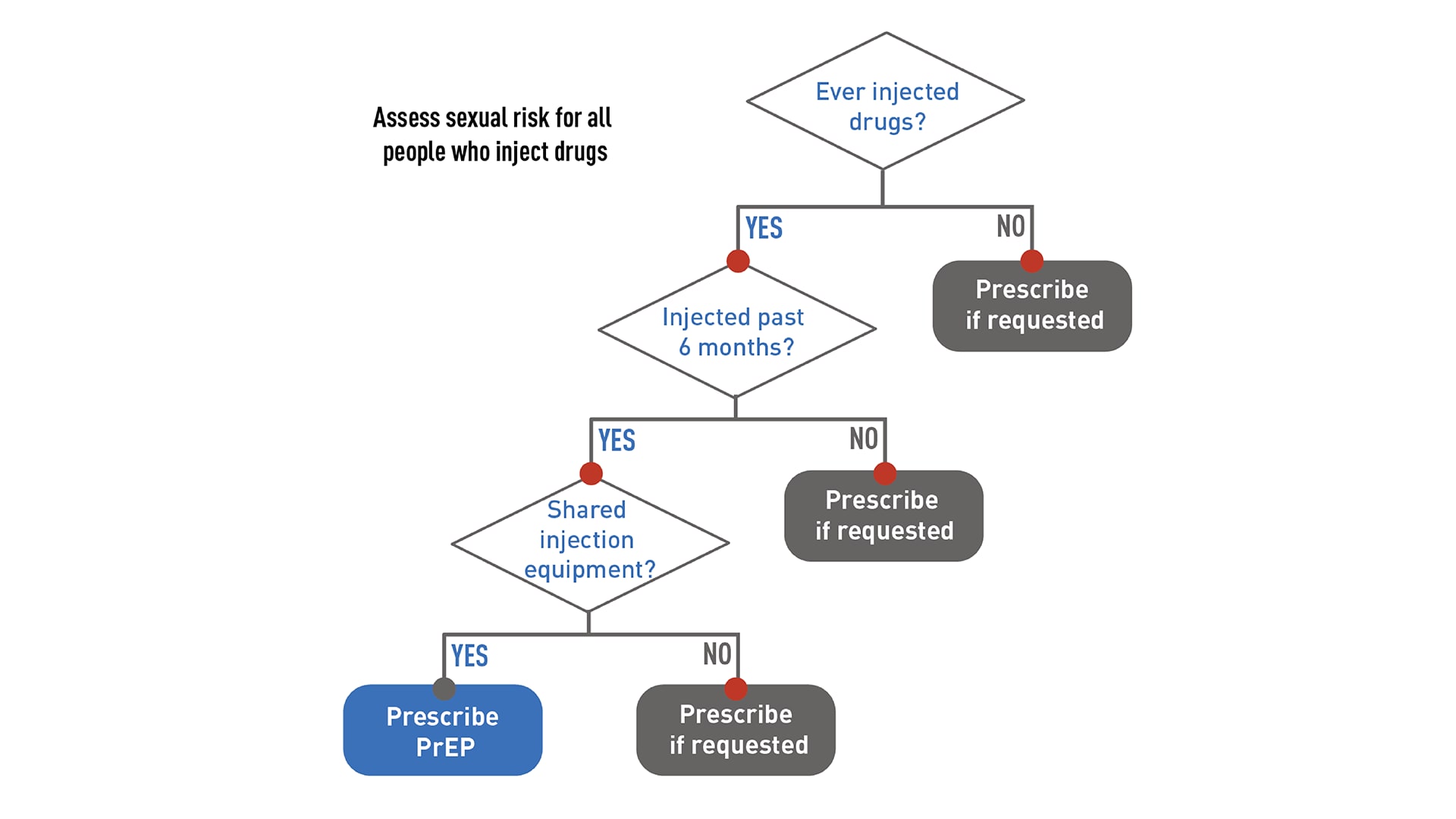
Offering PrEP to people who inject drugs
Evaluation
Before prescribing PrEP, perform the required baseline assessments.
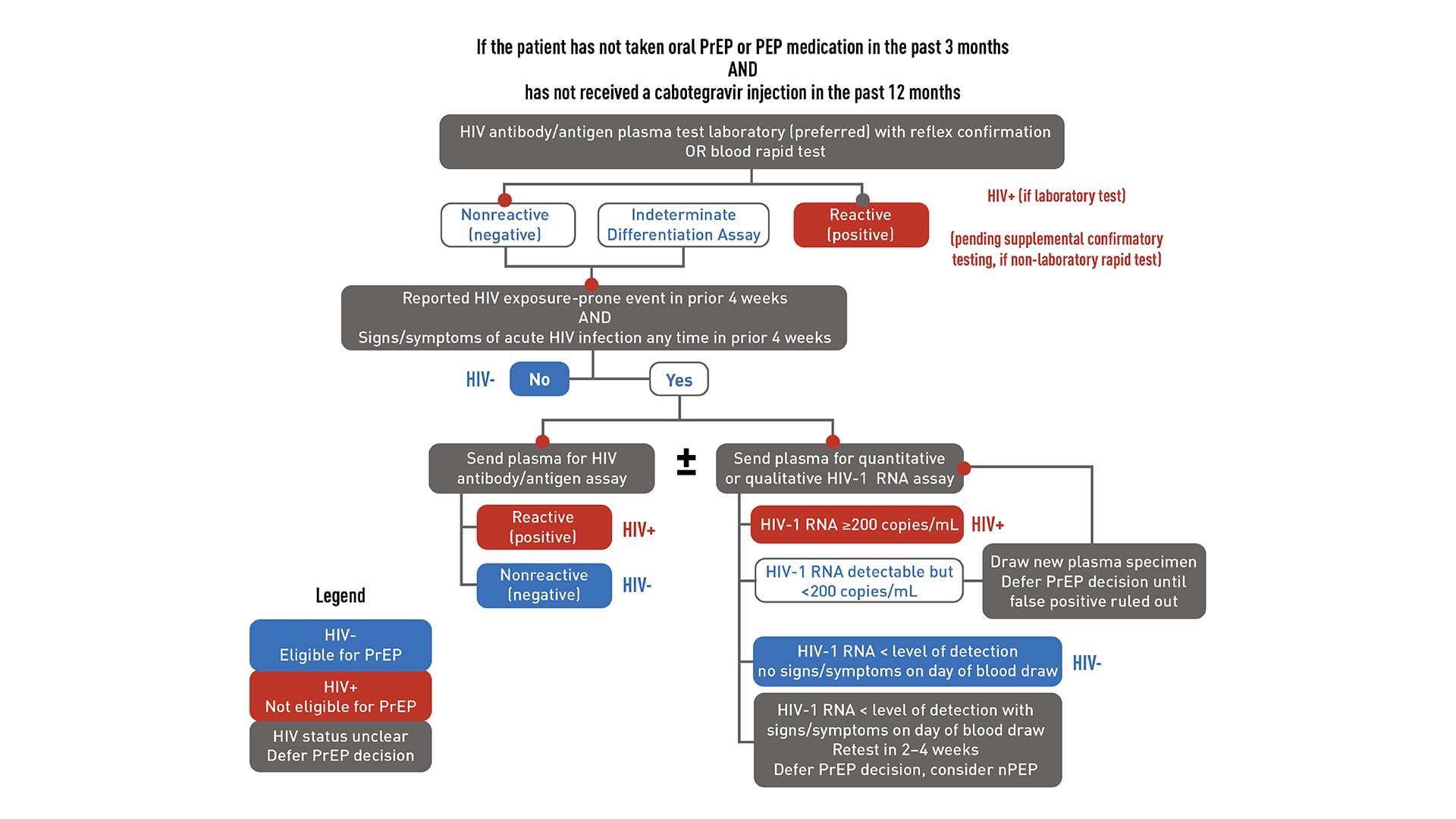
HIV testing is required to confirm that patients do not have HIV before starting PrEP. Required testing differs depending on recent antiretroviral PrEP or PEP (post-exposure prophylaxis) use. "Recent use" is defined as taking oral PrEP in the last 3 months or receiving a cabotegravir (CAB) injection in the last 12 months.
Download the flowcharts below to learn more about required HIV testing.
HIV testing for patients starting or restarting PrEP after a long stop
HIV testing for patients who are taking or have recently taken PrEP
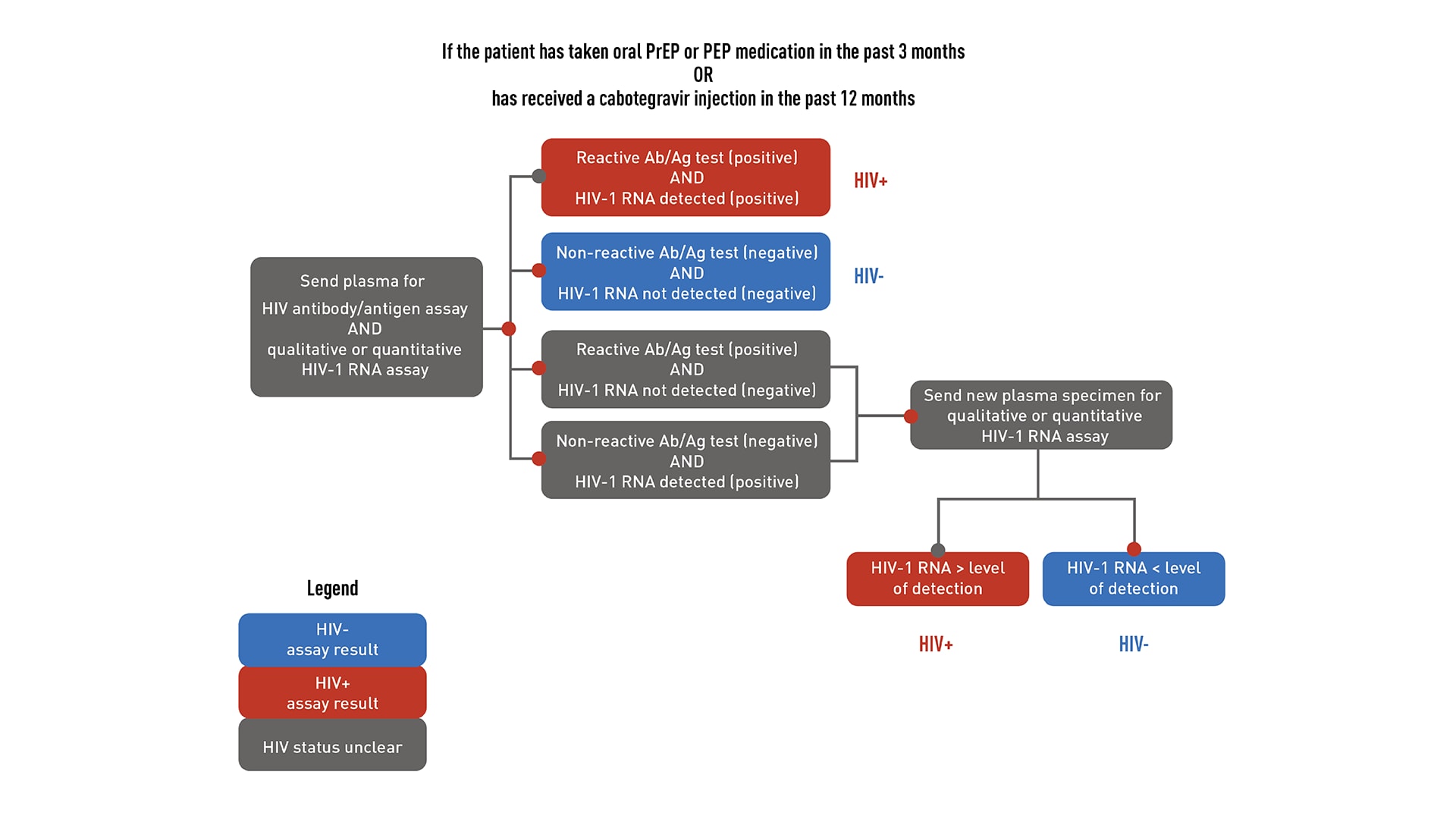
If a patient has a negative antigen/antibody test and an undetectable HIV-1 RNA test (if applicable) confirming they do not have HIV, PrEP can be prescribed. However, if a patient has both a positive antigen/antibody test and a detectable HIV-1 RNA test confirming that they have HIV, link that patient to HIV care and treatment. If results are discordant or ambiguous, obtain a new blood specimen for retesting, and do not prescribe PrEP until HIV status is confirmed.
You can draw blood, send it to a laboratory for testing, and wait for the results before prescribing or continuing PrEP; or you can use a rapid, point-of-care, FDA-approved fingerstick HIV antigen/antibody blood test and draw blood to send for laboratory testing. PrEP can be prescribed or continued based on a negative rapid antigen/antibody test result while awaiting laboratory test results.
Avoid using oral rapid tests to screen for HIV when considering offering or continuing PrEP because they are less sensitive than blood tests and may not detect recent HIV infection.7 A listing of FDA-approved HIV tests, specimen requirements, and window periods is available from CDC.
Since PrEP is indicated for people with risk factors for HIV, acute HIV infection should be suspected in patients who were recently exposed. Ask all PrEP candidates with a negative or indeterminate HIV test result about whether they have experienced any signs or symptoms of viral infection in the past month or on the day of evaluation.
If your patient reports signs or symptoms of acute HIV infection within the past month:
- Test the patient with a combination antibody/antigen assay.
- Ideally, use a laboratory-based method.
- If a point-of-care antigen/antibody test is non-reactive (negative), PrEP can be started while waiting for confirmatory laboratory test results.
- Ideally, use a laboratory-based method.
- Test the patient's HIV-1 RNA.
- If the patient has a measurable viral load that is <200 copies/mL, they may have HIV or a false-positive test result.
- PrEP should be deferred while testing is repeated on a new blood specimen.
- If the viral load is below the level of detection of the assay for the second blood specimen, and the patient has no signs or symptoms, the first HIV-1 RNA test result was a false positive. The patient does not have HIV, and PrEP can be started.
- If the patient has a measurable viral load that is <200 copies/mL, they may have HIV or a false-positive test result.
Tests to screen for chlamydia, gonorrhea, and syphilis are recommended for all sexually active adults before starting oral or injectable PrEP.
Kidney function
Oral PrEP
For patients taking F/TDF or F/TAF as PrEP, assess kidney function before starting PrEP.
When used as PrEP, tenofovir can cause decreases in kidney function that are generally small, usually remain within the normal range, and are of no known clinical significance.78 These small decreases typically return to earlier levels when the patient stops taking the medication.910 Occasional cases of acute kidney failure, including Fanconi's syndrome, have occurred.811121314 Therefore, all patients considered for oral PrEP must have their kidney function assessed at PrEP initiation and periodically thereafter so that oral PrEP can be stopped, if necessary.
Assess kidney function using the Cockcroft-Gault formula with the patient's serum creatinine value to calculate their estimated creatinine clearance (eCrCl):15
- F/TDF is approved for use in people with eCrCl >60 mL/min.
- F/TAF is approved for use in people with eCrCl ≥30 mL/min.
Injectable PrEP
Kidney assessments are not necessary for patients taking CAB.
HBV serology
Emtricitabine and tenofovir can be used to treat active hepatitis B virus (HBV) infection. However, in people with active HBV, stopping these medicines can result in a rebound of HBV replication leading to liver damage.
HBV infection is not a contraindication to PrEP, but all people considered for oral PrEP with F/TDF or F/TAF must be screened for HBV. Patients with active HBV infection should be educated about the risks of stopping oral PrEP without appropriate follow-up so that if they stop using oral PrEP, their liver function can be closely monitored for reactivation of HBV replication.
Lipid profile
For patients taking F/TAF as oral PrEP, assess cholesterol and triglyceride levels before starting PrEP.
Treatment options
Two consist of a combination of drugs in a single oral tablet taken daily. The third is a medication given by injection every 2 months.
- Emtricitabine (F) 200 mg in combination with tenofovir disoproxil fumarate (TDF) 300 mg (F/TDF—brand name Truvada® or generic equivalent).
- Emtricitabine (F) 200 mg in combination with tenofovir alafenamide (TAF) 25 mg (F/TAF—brand name Descovy®).
- Cabotegravir (CAB) 600 mg injection (brand name Apretude®).
These medications are approved to prevent HIV in adults and adolescents weighing at least 77 lb (35 kg) as follows:
- Daily oral PrEP with F/TDF is recommended to prevent HIV among all people with sex or injection drug use risk factors.
- Daily oral PrEP with F/TAF is recommended to prevent HIV through sexual transmission, excluding people likely to get HIV through receptive vaginal sex. F/TAF has not yet been studied for HIV prevention for people assigned female at birth who could get HIV through receptive vaginal sex.
- Injectable PrEP with CAB is recommended to prevent sexual transmission of HIV among all people. CAB is given as an intramuscular injection. CAB for PrEP is started by administering the first injection followed by a second injection 1 month after the first. CAB injections are given every 2 months thereafter.
When taken as prescribed, both oral161718 and injectable1920 PrEP reduce the risk of getting HIV from sex by about 99%. Oral PrEP has also been shown to reduce the risk of getting HIV from injection drug use by at least 74%, when taken as prescribed.2122
Complications
PrEP has not caused serious short- or medium-term safety concerns.16192324
Oral PrEP
F/TDF as PrEP is considered generally safe for people who are pregnant or breast/chestfeeding.25 If a patient who is or may become pregnant has concerns, discuss those concerns with the patient. Then, decide together if the chances of getting HIV through sex or injection drug use is sufficiently high to use PrEP, knowing that pregnancy is associated with an increased risk of getting HIV.26
Daily oral PrEP with F/TAF is recommended to prevent HIV through sex, excluding people who have receptive vaginal sex. F/TAF has not yet been studied for HIV prevention for receptive vaginal sex.
Because F/TDF and F/TAF are eliminated by the kidneys, prescribe these PrEP medications only to patients without severe kidney impairment. Co-administer oral PrEP with care in patients taking other drugs eliminated by the kidneys. Examples of drugs eliminated by the kidneys include acyclovir, adefovir dipivoxil, cidofovir, ganciclovir, valacyclovir, valganciclovir, aminoglycosides, and high-dose or multiple nonsteroidal anti-inflammatory drugs (NSAIDs). Drugs that decrease kidney function may also increase serum concentrations of tenofovir or emtricitabine.2527 Instead of oral PrEP, CAB injections are recommended for people who have serious kidney disease with eCrCl <30 mL/min.
Injectable PrEP
In clinical trials, injection site reactions (e.g., pain, tenderness, local skin swelling) were frequently reported following CAB injections.28 Patients should be informed that these reactions are common, generally mild or moderate, and do not last long.
Although there are PrEP medication and dosing options to meet patients' differing needs, PrEP isn't right for some patients.
People with HIV should not take PrEP
- Health care providers must confirm that patients do not have HIV before starting PrEP.
- Excluding people with acute HIV infection is critically important so that they do not develop drug-resistant HIV because they took PrEP medication while in the early stages of HIV.
- F/TDF and F/TAF are appropriate parts of a regimen to treat HIV but must be combined with additional antiretrovirals to provide effective treatment.
- CAB must be combined with other antiretroviral medication to provide effective treatment for people with HIV.
Oral PrEP
Oral PrEP isn't the right choice for people with severe kidney impairment. Before prescribing oral PrEP, providers should confirm that each patient's eCrCl is high enough, using the Cockcroft-Gault formula:
- ≥60 mL/min for F/TDF or F/TAF
- ≥30 mL/min for F/TAF
2-1-1 dosing of oral PrEP
2-1-1 dosing of oral PrEP is also known as event-driven, intermittent, on-demand, or coitally timed PrEP. Although this dosing option can be used by adult men who have sex with men (MSM) prescribed F/TDF, it is not approved by the FDA and is not recommended by CDC.
It is not right for:
- People other than adult MSM. The 2-1-1 dosing method has only been studied in adult MSM.
- People with active HBV infection. Episodic F/TDF exposure can cause hepatic flares in people with active HBV infection and should be avoided.
Injectable PrEP
Injectable PrEP is not right for people with sensitivity to CAB. CAB should not be given to people with a history of hypersensitivity reaction to CAB.
Access CDC's comprehensive guidelines for prescribing PrEP in A Clinical Practice Guideline for PrEP29 and the Clinical Providers' Supplement for PrEP.25
The Clinical Providers' Supplement for PrEP contains additional tools for clinicians providing PrEP, such as
- Patient/provider checklists
- Patient information sheets
- Provider information sheets
- HIV risk screening assessments for gay, bisexual, and other MSM and people who inject drugs
- Supplemental counseling information
- Billing codes
- Practice quality measures
Managing patients on PrEP
Offer PrEP medication and dosing options to meet patients' needs.
High adherence to daily oral PrEP is required for it to be effective.161728 A few cases of HIV infection have been reported among gay, bisexual, and other men who have sex with men whose high adherence to PrEP was verified. These rare cases show that the risk of HIV when oral PrEP is taken as prescribed is extremely low.
Based on existing research, oral PrEP reaches maximum drug levels associated with protection from HIV for receptive anal sex at about 7 days of daily use. For receptive vaginal sex and injection drug use, oral PrEP reaches maximum drug levels at up to about 21 days of daily use. There are no data available about how long it takes for PrEP to reach protective levels for insertive vaginal and anal sex.
Oral PrEP with emtricitabine/tenofovir disoproxil fumarate (F/TDF) can be prescribed off-label using "2-1-1" dosing for adult MSM. This is also known as event-driven, intermittent, on-demand, or coitally timed PrEP. When using 2-1-1 dosing, the patient takes F/TDF doses based on when they plan to have sex: two pills 2-24 hours before sex, one pill 24 hours after the first two-pill dose, and one pill 48 hours after the first two-pill dose.
Off-label 2-1-1 dosing is not approved by the FDA and not recommended by the Centers for Disease Control and Prevention (CDC). However, it can be prescribed to MSM who meet the following criteria:
- Request non-daily dosing
- Have sex infrequently (e.g., less often than once a week)
- Can anticipate sex (or delay sex) to permit the doses at least 2 hours prior to sex
2-1-1 dosing should not be prescribed for MSM who might have problems adhering to a complex dosing regimen (e.g., adolescents, patients with an active substance use disorder).
Injectable PrEP may be appropriate for people who have problems taking oral PrEP as prescribed.
When taken as prescribed, injectable PrEP with cabotegravir (CAB) is over 99% effective at reducing the risk of getting HIV from sex.1920 CAB injections may be especially useful for people who:
- Have problems taking oral PrEP as prescribed
- Prefer getting a shot every 2 months instead of taking oral PrEP
- Have serious kidney disease that prevents use of oral PrEP medications
CAB is given as an intramuscular injection initiated as a first injection followed by a second injection 1 month after the first. Injections continue every 2 months thereafter. A 4-week lead-in period of 30 mg daily oral CAB prior to the first injection is optional for patients who are worried about side effects.
Returning for bimonthly injections is necessary to maintain protective levels of medication. In trials, HIV infections have occurred among people who delayed receiving their injections for several months. There are no available data for CAB to estimate how long it takes to reach maximal protection against HIV.
Prescribe PrEP as part of a combination prevention plan. At minimum, while patients are on PrEP, CDC guidelines recommend that health care providers implement support and ongoing assessments.
- Repeat HIV antigen/antibody and HIV-1 RNA tests and assess for signs or symptoms of acute HIV infection to confirm that patients do not have HIV.
- Provide a prescription or refill authorization of daily oral PrEP medication for no more than 90 days (until the next HIV test).
- Assess and provide support for medication adherence and risk-reduction behaviors.
- Test sexually active patients with signs or symptoms of sexually transmitted infections (STIs). Screen asymptomatic MSM with certain risk factors for recurrent bacterial STIs (oral, rectal, urine, blood). Examples of MSM who should be screened include those with syphilis, gonorrhea, or chlamydia at prior visits or multiple sex partners.
- Provide access to sterile needles/syringes and substance use disorder treatment services for people who inject drugs.
- Respond to new questions and provide any new information about PrEP use.
- Monitor estimated creatinine clearance (eCrCl) for patients age ≥50 years or who had an eCrCl <90 mL/min when they started oral PrEP.
- If there are other threats to kidney safety (e.g., hypertension, diabetes), kidney function may need to be monitored more often or checked using additional tests (e.g., urinalysis for proteinuria).
- A rise in serum creatinine is not a reason to withhold PrEP if eCrCl remains ≥60 mL/min for F/TDF or ≥30 mL/min for emtricitabine/tenofovir alafenamide (F/TAF).
- If eCrCl is declining steadily (but still ≥60 mL/min for F/TDF or ≥30 mL/min for F/TAF), consult with a nephrologist, if needed, or evaluate other possible threats to kidney health.
- Screen sexually active people for STIs (vaginal, oral, rectal, urine, as indicated; blood): syphilis and gonorrhea for all PrEP users; chlamydia for MSM and transgender women, even if asymptomatic.
- Assess interest in continuing or stopping PrEP.
- Monitor eCrCl for all patients continuing oral PrEP medication.
- Monitor triglyceride and cholesterol levels and weight for patients prescribed F/TAF for PrEP.
- Screen heterosexually active people for chlamydia (vaginal, urine), even if asymptomatic.
For patients on injectable PrEP
- Test for HIV with antigen/antibody and HIV-1 RNA assays and assess for signs or symptoms of acute infection.
- Administer CAB injection.
- Respond to new questions.
- Provide medication adherence and behavioral risk-reduction support.
- Test for HIV with antigen/antibody and HIV-1 RNA assays and assess for signs or symptoms of acute infection.
- Administer CAB injection.
- Provide access to sterile needles/syringes and substance use disorder treatment services for people who inject drugs.
- Respond to new questions and provide any new information about CAB for PrEP.
- Discuss the benefits of persistent CAB for PrEP use and adherence to schedule injection visits.
- Conduct bacterial STI screening for MSM and transgender women who have sex with men (oral, rectal, urine, blood).
- Screen all heterosexually active people for bacterial STIs (vaginal, rectal, urine, as indicated; blood).
- Assess desire to continue PrEP injections and screen heterosexually active people for chlamydia (vaginal, urine), even if asymptomatic.
Oral PrEP
Discuss with patients how to safely discontinue and restart daily PrEP both when starting PrEP and when discontinuing PrEP. Protection from HIV will wane over 7-10 days after ceasing daily oral PrEP use. Because some patients can acquire HIV soon after stopping PrEP, providers should assess ongoing risk factors for HIV and discuss other prevention methods if HIV exposure is anticipated, including nonoccupational post-exposure prophylaxis (PEP).
Injectable PrEP
CAB levels slowly wane over many months after injections are discontinued. At some point during this "tail period," CAB levels will fall below a protective threshold and persist for some time at nonprotective levels. For these reasons, patients discontinuing CAB injections who may have ongoing risk factors should be provided with another highly effective HIV prevention method during the months following their last injection.
Providers should follow these steps when a patient discontinues or misses CAB injections:
- Counsel patient about the risk of developing drug-resistant HIV during declining CAB levels (the "tail period") after stopping or missing CAB injections.
- Assess ongoing chances of HIV exposure and prescribe daily oral PrEP within 8 weeks after the last CAB injection or other prevention methods if HIV exposure is anticipated, including nonoccupational PEP).
- Continue follow-up visits quarterly for 12 months. Conduct antigen/antibody and HIV-1 RNA tests at each quarterly follow-up visit after stopping CAB injections.
For more information about the "tail period" after stopping CAB injections, see page 53 of A Clinical Practice Guideline for PrEP.
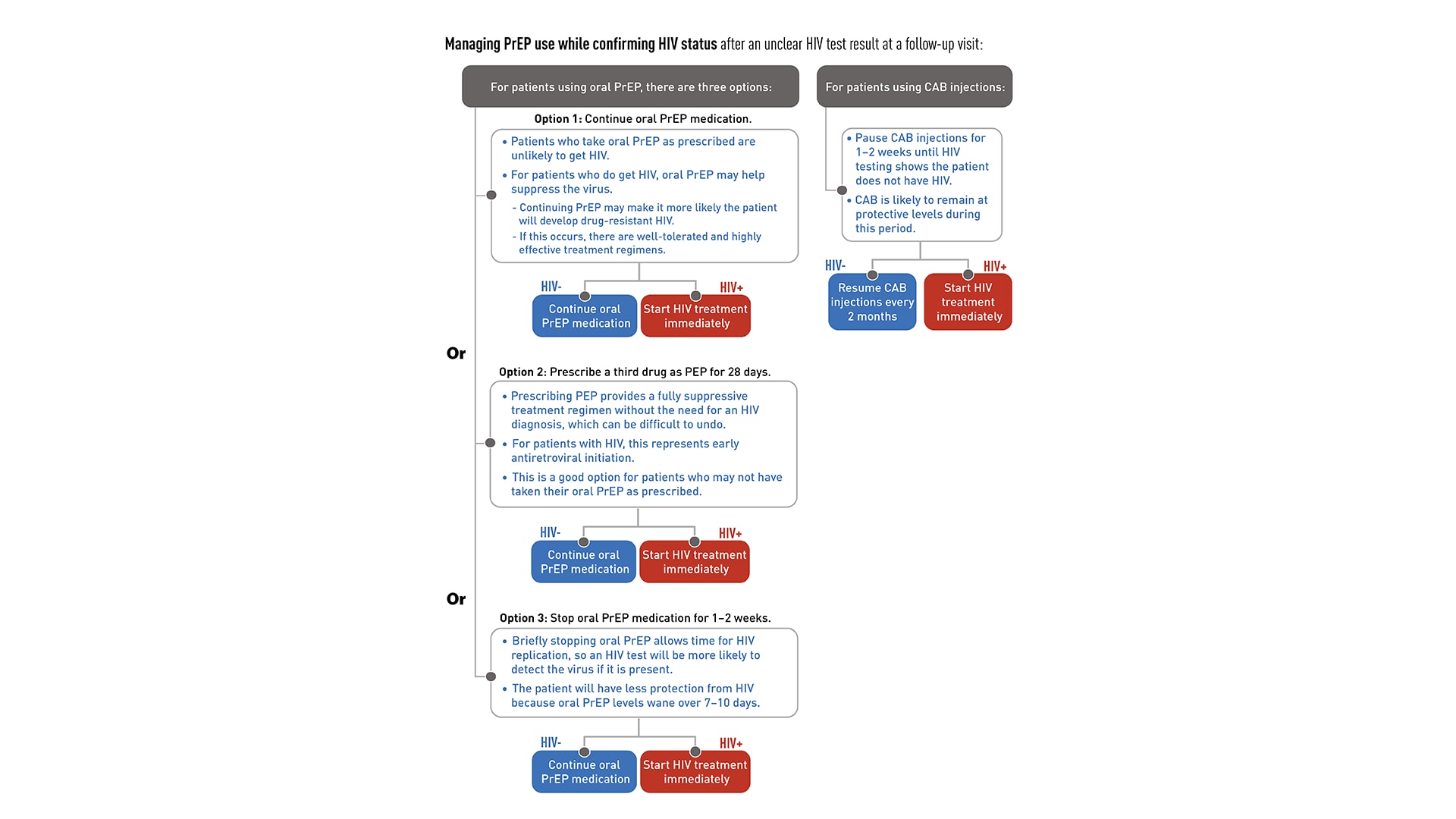
If a patient has unclear HIV test results at a follow-up visit, providers can follow these steps to confirm the patient's true HIV status:
- Wait 5-7 days and then draw a new blood specimen for repeat laboratory HIV testing using both an HIV antigen/antibody test and an HIV-1 RNA assay.
- If the repeat testing results show the patient has HIV, link the patient to HIV care and treatment. If the repeat testing results confirm that the patient does not have HIV, continue PrEP.
- If the results are still ambiguous, contact the National Clinician Consultation Center PrEPline at 1-855-448-7737 (Monday–Friday, 9:00 AM–8:00 PM ET) to get further testing advice and for linkage to a laboratory that can do specialized testing to confirm their HIV status.
While HIV status is being confirmed, providers have the following antiretroviral management options:
For patients using oral PrEP:
Continue oral PrEP medication:
- Because PrEP is highly effective, it is unlikely that a patient who takes PrEP medication as prescribed will get HIV.
- If the patient does have HIV, continuing PrEP may help to suppress the virus but may also increase the probability that the patient will develop drug-resistant HIV.
- If this occurs, there are well-tolerated and highly effective treatment regimens available.
Prescribe a third drug as PEP for 28 days:
- This option provides a fully suppressive treatment regimen without the need to diagnose the patient with HIV, which can be difficult to undo if further testing shows the patient does not have HIV.
- If the patient is found to have HIV, this regimen can be considered early antiretroviral initiation and be continued.
- This option is especially applicable for patients who may not have taken their daily oral PrEP medication as prescribed.
Stop oral PrEP medication for 1-2 weeks:
- If the patient has HIV, briefly stopping PrEP allows time for HIV replication to occur and increases the likelihood that an HIV test will detect the virus, if it is present.
- Note that stopping oral PrEP will leave the patient with less protection from HIV because oral PrEP levels wane over 7-10 days after the medication is stopped.
For patients using CAB injections:
- Pause CAB injections until testing shows that the patient does not have HIV.
- During the 1-2 weeks needed for additional HIV testing to determine HIV status, CAB is likely to remain at protective levels.
- If the patient has HIV, start HIV treatment immediately.
- If the patient does not have HIV, resume CAB injections every 2 months.
Managing PrEP use while confirming HIV status after an ambiguous HIV test result
Once additional laboratory tests have confirmed the patient has HIV, take the following steps:
- Initiate treatment or refer for comprehensive HIV care.
- Counsel the patient about how to prevent HIV transmission to others and improve their own health.
- Report the new HIV infection to the local health department.
The recent expansion of telehealth care to replace some or all in-clinic visits has led to adaptations for prescribing PrEP. These adaptations can include the following procedures:
- Providers may conduct PrEP screening, initiation, or follow-up visits by phone or web-based consult with patients.
- Regular HIV testing should be continued for patient safety.
- Lab-only visits for HIV testing and other indicated tests for the provision of PrEP are strongly preferred.
- Only in situations where no laboratory or clinic visits are possible (e.g., local emergencies or lock-downs), patients may conduct self-testing using an oral swab-based test. This type of HIV test is usually not recommended for PrEP patients because it may not detect recent HIV infection in people taking PrEP medications.
- Lab-only visits for HIV testing and other indicated tests for the provision of PrEP are strongly preferred.
- After confirming patient does not have HIV, if prescribing oral PrEP, providers can consider writing a prescription for a 90-day supply of medication rather than a 30-day supply with two refills. This will help patients minimize trips to the pharmacy and facilitate oral PrEP adherence.
Considerations for specific populations
Oral PrEP with emtricitabine/tenofovir disoproxil fumarate (F/TDF, brand name Truvada® or generic equivalent) can be prescribed off-label using "2-1-1" dosing for adult MSM. Off-label 2-1-1 dosing is not approved by the US Food and Drug Administration (FDA) and not recommended by the Centers for Disease Control and Prevention (CDC). However, it can be prescribed to MSM who meet the following criteria:
- Request non-daily dosing.
- Have sex infrequently (e.g., less often than once a week).
- Can anticipate sex (or delay sex) to permit the doses at least 2 hours prior to sex.
Transgender and nonbinary people who use gender-affirming hormones and have HIV risk factors can use PrEP. There are no known drug conflicts between PrEP and hormone therapy, and there is no reason why the drugs cannot be taken at the same time.
There are three PrEP regimens approved for transgender women and nonbinary people assigned male at birth:
- Daily oral F/TDF
- Daily oral F/TAF
- CAB injections every two months
There are two PrEP regimens approved for transgender men and nonbinary people assigned female at birth who may have receptive vaginal sex:
- Daily oral F/TDF
- CAB injections every two months
- Emtricitabine with tenofovir alafenamide (F/TAF, brand name Descovy®) has not yet been studied for HIV prevention among people assigned female at birth who could get HIV through receptive vaginal sex
There are two PrEP regimens approved for use by cisgender women:
- Daily oral F/TDF
- CAB injections every two months
Emtricitabine with tenofovir alafenamide (F/TAF, brand name Descovy®) has not yet been studied for HIV prevention among cisgender women who could get HIV through receptive vaginal sex.
PrEP and birth control
PrEP medications that are approved for use by people with the potential for pregnancy can be used with birth control. There are no known interactions between PrEP and hormone-based birth control methods, such as the pill, patch, ring, shot, implant, and intrauterine device (IUD).
PrEP and HIV during conception, pregnancy, and breast/chestfeeding
There are two recommended PrEP regimens for people who may become pregnant:
- Oral PrEP. F/TDF as PrEP is considered generally safe for people who are pregnant or breast/chestfeeding.303132 Current perinatal antiretroviral treatment guidelines recommend PrEP with T/TDF for people who may become pregnant, those who are pregnant, and those who are breast/chestfeeding.253033
- Injectable PrEP. Currently, the published data on CAB-exposed pregnancies among pregnant people without HIV are sparse.31 CAB injections may be initiated or continued in people who may become pregnant if the patient and provider agree that the benefits outweigh potential risks.
Concerns around fetal and infant exposure to PrEP medications
Discuss PrEP and other HIV prevention methods with all your patients who may become pregnant, including potential risks and benefits during conception, pregnancy, and breast/chestfeeding.32 If a patient who is or may become pregnant has concerns, talk about those concerns with the patient. Then, decide together if the chances of getting HIV through sex or injection drug use are sufficiently high to use PrEP, knowing that pregnancy is associated with an increased chance of getting HIV.2634
The Antiretroviral Pregnancy Registry confidentially collects data on prenatal and perinatal exposure to PrEP medications. The goal of this registry is to provide early warning of any negative effects of these medications on fetal and infant development. Providers are strongly encouraged to submit information prospectively and anonymously about any pregnancies in which PrEP is used to the Antiretroviral Pregnancy Registry website.
People who inject drugs that are not prescribed to them should be offered PrEP. Oral PrEP has been shown to reduce the risk of getting HIV from injection drug use by at least 74%, when taken as prescribed.2122 The US Preventive Services Task Force's grade IA recommendation for prescribing PrEP with cabotegravir (CAB, brand name Apretude®) states that CAB is recommended for adults who may get HIV through sex. Because most people who inject drugs are also sexually active, they should be assessed for sexual risk factors and provided the option of CAB for PrEP when indicated.
Substance use disorder treatment and behavioral health services
Provide your patients who inject drugs and use PrEP with access to substance use disorder treatment services during regular follow-up appointments.
For patients on oral PrEP, provide access at least every 3 months. For patients on injectable PrEP, provide access at least every 2 months (beginning in month 3).
Assess your patients who inject drugs to identify needs for cognitive or behavioral counseling or mental health or social services. Providing or referring your patients to these services can help them manage their HIV risk factors.
Offer medication-assisted treatment to your patients who inject opioids. This can be done within the PrEP clinical setting (e.g., through the provision of daily oral buprenorphine or naltrexone) or by referral to a substance use disorder treatment clinic (e.g., methadone program). Find local substance use disorder treatment resources online on the Substance Abuse and Mental Health Services' Locator Map.
Access to sterile injection equipment
Not all patients who inject drugs are able or ready to engage in substance use treatment. Provide these patients with access to sterile injection equipment to help reduce their exposure to HIV and other infectious agents (e.g., hepatitis C). This should be done as part of your regular follow-up appointments with patients using PrEP:
- For patients on oral PrEP: Provide access at least every 3 months.
- For patients on injectable PrEP: Provide access at least every 2 months (beginning in month 3).
- You can use the following methods to provide access to sterile injection equipment:
- Link patients to syringe service programs, if they are legal and available in your area.
- Prescribe syringes.
- Advise patients to purchase syringes from pharmacies without a prescription, if it is legal in your area.
- Link patients to syringe service programs, if they are legal and available in your area.
PrEP is recommended for adolescents without HIV who weigh at least 77 pounds (35 kilograms). While there has been less research on PrEP use among adolescents, available data suggest that PrEP is safe and effective for HIV prevention among adolescent patients.35
- Daily oral F/TDF and F/TAF are FDA-approved PrEP options for adolescents.
- F/TAF has not yet been studied for HIV prevention for people assigned female at birth who could get HIV through receptive vaginal sex.
- CAB has not been studied in men or women <18 years of age. These studies are underway. However, until CAB is determined to be safe for this population and approved by the FDA, CAB injections are not recommended for adolescents <18 years old.
Taking a sexual and substance use history is essential to understanding your adolescent patients' HIV risk factors and whether PrEP is right for them. Providers should offer PrEP to any adolescent who reports HIV risk factors relating to sex or injecting drugs.
Legal considerations
Laws and regulations that may be relevant for PrEP-related services provided to adolescent minors (including HIV testing) differ by jurisdiction. Such laws and regulations may affect consent, confidentiality, parental disclosure, and circumstances requiring reporting to local agencies. When considering providing PrEP to an adolescent <18 years old, consult local laws, regulations, and policies that may apply.
Adolescents may be concerned about confidentiality, which can prevent them from seeking the support they need. To build trust with adolescent patients, explicitly discuss confidentiality based on local laws and policies and the methods you will use to ensure confidentiality is maintained to the extent permitted. Visit CDC's page on relevant laws and policies to learn more.
If the law permits, allow adolescent patients who would benefit from PrEP to decide for themselves whether to take it. When it is safe and appropriate, encourage them to include others, such as parents and guardians, in the conversation about PrEP.35
Talk to your adolescent patients about the importance of adhering to PrEP. PrEP must be taken as prescribed for it to work. Talk with your adolescent patients about the importance of taking their medication as prescribed and strategies to help them maintain medication adherence. Because adolescents often experience difficulties with adherence, your adolescent patients on PrEP may benefit from more frequent, supportive interactions.363738
Bone density changes
Data have shown bone density changes in young MSM during PrEP use and after completing a PrEP trial period of 48 weeks. Findings indicate decreased bone mineral density during the use of F/TDF as PrEP, with larger declines in those ages 15–19 years than in those ages 20–22 years. While men ages 18–22 years had full improvement during the 48 weeks after PrEP use stopped, declines persisted in younger men.39
These bone density changes were more frequently seen in young men with greatest adherence (i.e., highest drug exposure).39 Because differences in pharmacodynamics suggest less bone effect with F/TAF than with F/TDF, consider prescribing F/TAF to adolescent male patients initiating PrEP. The likelihood of long-term bone health should be weighed against the potential benefit of providing PrEP for an individual adolescent with HIV risk factors.
Kidney function must be considered when working with patients to select an appropriate PrEP regimen. Assess your patients' kidney function before they start oral PrEP and then at regular follow-up appointments.
Kidney function assessments are not needed for patients receiving CAB injections.
People who have sexual partners with HIV
Patients without HIV whose sexual partners have HIV can use PrEP for HIV prevention. CAB injections or daily oral PrEP are recommended options for these patients, especially if the patient's partner or partners may not be virally suppressed.
When assessing indications for PrEP use in mixed-HIV-status sexual partners, ask about the treatment and viral load status of the partner with HIV. People with HIV who achieve and maintain a plasma HIV RNA viral load <200 copies/mL with antiretroviral therapy won't transmit HIV.40 This is sometimes referred to as Undetectable=Untransmittable (U=U) or treatment as prevention.41
PrEP may be indicated for the partner without HIV if any of the following criteria are met:
- The partner with HIV has been inconsistently virally suppressed or their viral load status is unknown.
- The partner without HIV has other sexual partners, especially if their HIV statuses are unknown.
- The partner without HIV wants the additional reassurance of protection that PrEP can provide.
PrEP should not be withheld from patients without HIV who request it, even if their sexual partner with HIV is virally suppressed. Several studies using viral genotyping have documented instances where a person without HIV acquired HIV from having sex with a person with HIV outside the relationship with the partner known to have HIV.42
Connecting patients with PrEP resources
PrEP is more accessible than ever. The US Preventive Services Task Force issued a Grade A recommendation for PrEP. This means most private insurance and Medicaid programs are required to cover PrEP services without cost sharing, like copays or deductibles. Prior authorization may be required.
Additionally, some states have their own PrEP assistance programs. Some cover medication, some cover clinical visit and lab costs, and some cover both. To learn more, consult NASTAD's list of state PrEP assistance programs.
The Gilead Sciences Advancing Access program offers eligible patients assistance with medication costs for oral PrEP.
ViiVConnect offers a medication assistance program to help patients pay for CAB injections.
Resources
Expert Consultation Services
Materials for you and your practice
Materials for your patients
- Qiao S, Zhou G, Li X. Disclosure of same-sex behaviors to health-care providers and uptake of HIV testing for men who have sex with men: a systematic review. Am J Mens Health. 2018;12(5):1197-1214. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6142161/
- Petroll AE, Mosack KE. Physician awareness of sexual orientation and preventive health recommendations to men who have sex with men. Sex Transm Dis. 2011;38(1):63. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4141481/
- US Preventive Services Task Force. Screening for Illicit Drug Use. Accessed August 20, 2013, https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening
- McDonell MG, Graves MC, West II, et al. Utility of point of care urine drug tests in the treatment of primary care patients with drug use disorders. J Addict Med. 2016;10(3):196. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880495/
- Chasnoff IJ, Landress HJ, Barrett ME. The prevalence of illicit-drug or alcohol use during pregnancy and discrepancies in mandatory reporting in Pinellas County, Florida. N Engl J Med. 1990;322(17):1202-1206. https://www.nejm.org/doi/full/10.1056/NEJM199004263221706
- White D, Rosenberg ES, Cooper HL, et al. Racial differences in the validity of self-reported drug use among men who have sex with men in Atlanta, GA. Drug Alcohol Depend. 2014;138:146-153. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4104302/
- Martin M, Vanichseni S, Suntharasamai P, et al. Renal function of participants in the Bangkok tenofovir study—Thailand, 2005–2012. Clin infect Dis. 2014;59(5):716-724. https://academic.oup.com/cid/article/59/5/716/2895408?login=true
- Mugwanya KK, Wyatt C, Celum C, et al. Changes in glomerular kidney function among hiv-1-uninfected men and women receiving emtricitabine–tenofovir disoproxil fumarate preexposure prophylaxis: a randomized clinical trial. JAMA Intern Med. 2015;175(2):246-254. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4354899/
- Emtricitabine and tenofovir disoproxil fumarate. Prescribing information. Teva Pharmaceuticals Inc.; 2020. Accessed January 7, 2022. https://www.tevahivgenerics.com/globalassets/emtricitabine/pdfs/tg-42450_emtricitabine-and-tenofovir-disoproxil-fumarate-tablets-promo-pi-for-use-electronically.pdf
- Truvada. Prescribing information. Gilead Sciences; 2020. Accessed January 7, 2022. https://www.gilead.com/-/media/files/pdfs/medicines/hiv/truvada/truvada_pi.pdf
- Antoni G, Tremblay C, Charreau I, et al. On-demand PrEP with TDF/FTC remains highly effective among MSM with infrequent sexual intercourse: a sub-study of the ANRS IPERGAY trial. Presented at: IAS Conference on HIV Science; July 23-27, 2017; Paris, France.
- Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320(4):379-396. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6415748/
- Mugwanya KK, Wyatt C, Celum C, et al. Changes in glomerular kidney function among HIV-1-uninfected men and women receiving emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis: a randomized clinical trial. JAMA Intern Med. 2015;175(2):246-54. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4354899/
- Tang EC, Vittinghoff E, Anderson PL, et al. Changes in Kidney Function Associated With Daily Tenofovir Disoproxil Fumarate/Emtricitabine for HIV Preexposure Prophylaxis Use in the United States Demonstration Project. J Acquir Immune Defic Syndr. 2018;77(2):193-198. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5762266/
- Wassner C, Bradley N, Lee Y. A review and clinical understanding of tenofovir: tenofovir disoproxil fumarate versus tenofovir alafenamide. J Int Assoc Provid AIDS Care. 2020;19:2325958220919231. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7163232/
- Grant RM, Anderson PL, McMahan V, et al. Update of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820-829. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6107918/
- Castillo-Mancilla JR, Zheng J-H, Rower JE, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retrovir. 2013;29(2):384-390. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3552442/
- Mayer KH, Molina, J-M, Thompson, MA, et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomized, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet. 2020;396(10246):239-254. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9665936/
- Landovitz RJ, Donnell D, Clement ME, et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med. 2021;385(7):595-608. https://www.nejm.org/doi/full/10.1056/NEJMoa2101016
- HPTN 084 study demonstrates superiority of CAB LA to oral TDF/FTC for the prevention of HIV. HPTN: HIV Prevention Trials Network; November 9, 2020. Accessed May 3, 2022. https://www.hptn.org/news-and-events/press-releases/hptn-084-study-demonstrates-superiority-of-cab-la-to-oral-tdfftc-for
- Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;381(9883):2083-2090. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(13)61127-7/fulltext
- Martin M, Vanichseni S, Suntharasamai P, et al. The impact of adherence to preexposure prophylaxis on the risk of HIV infection among people who inject drugs. AIDS. 2015;29(7):819-824 https://journals.lww.com/aidsonline/Fulltext/2015/04240/The_impact_of_adherence_to_preexposure_prophylaxis.8.aspx.
- Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399-410. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3770474/
- Donnell D, Baeten JM, Bumpus NN, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immun Def Syndr. 2014;66: 340-348. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4059553/
- Centers for Disease Control and Prevention, US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update: clinical providers' supplement. Accessed January 7, 2022. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-provider-supplement-2021.pdf
- Thompson KA, Hughes J, Baeten JM, et al. Increased risk of female HIV-1 acquisition throughout pregnancy and postpartum: a prospective per-coital act analysis among women with HIV-1 infected partners. J Infect Dis. 2018;218:16-25. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5989601/
- US Preventive Services Task Force. Preexposure prophylaxis for the prevention of HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321(22):2203-2213. https://jamanetwork.com/journals/jama/fullarticle/2735509
- Cottrell ML, Yang KH, Prince HMA, et al. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis. 2016;214(1):55-64. https://academic.oup.com/jid/article/214/1/55/2469748
- Centers for Disease Control and Prevention, US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update: a clinical practice guideline. Accessed January 7, 2022. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
- Teva Pharmaceuticals. Emtricitabine and tenofovir disoproxil fumarate tablets package insert. Published 2020. Accessed August 23, 2021. https://www.tevahivgenerics.com/globalassets/emtricitabine/pdfs/tg-42450_emtricitabine-and-tenofovir-disoproxil-fumarate-tablets-promo-pi-for-use-electronically.pdf
- Tolley EE, Zangeneh SZ, Chau G, et al. Acceptability of long-acting injectable cabotegravir (CAB LA) in HIV-uninfected individuals: HPTN 077. AIDS Behav. 2020;24(9):2520-2531. https://www.ncbi.nlm.nih.gov/ pmc/articles/PMC7423859/
- Lampe MA, Smith DK, Anderson GJE, et al. Achieving safe conception in HIV-discordant couples: the potential role of oral preexposure prophylaxis (PrEP) in the United States. American Journal of Obstet Gynecol. 2011;204(6):e1-e8. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5514a1.htm
- Gilead Sciences. Truvada package insert. Published 2016. Accessed August 8, 2016. https://www.gilead.com/~/media/files /pdfs/medicines/hiv/truvada/truvada_pi.pdf
- Mugo NR, Heffron R, Donnell D, et al. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1 serodiscordant couples. AIDS. 2011;25(15):1887-1895. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3173565/
- Tanner MR, Miele P, Carter W, et al. Preexposure prophylaxis for prevention of HIV acquisition among adolescents: clinical considerations, 2020. MMWR Recomm Rep. 2020:69(3):1-12. https://www.cdc.gov/mmwr/volumes/69/rr/rr6903a1.htm
- Myers JJ, Dufour M-SK, Koester KA, et al. Adherence to PrEP among young men who have sex with men participating in a sexual health services demonstration project in Alameda County, California. J Acquir Immune Defic Syndr. 2019;81(4):406-413. https://journals.lww.com/jaids/Fulltext/2019/08010/Adherence_to_PrEP_Among_Young_Men_Who_Have_Sex.7.aspx
- Gray WN, Netz M, McConville A et al. Medication adherence in pediatric asthma: A systematic review of the literature. Pediatr Pulmonol. 2018;53(5):668-684 https://onlinelibrary.wiley.com/doi/10.1002/ppul.23966
- Venditti EM, Tan K, Chang N, et al. Barriers and strategies for oral medication adherence among children and adolescents with Type 2 diabetes. Diabetes Res Clin Pract. 2018;139:24-31. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5955779/
- Havens PL, Perumean-Chaney SE, Patki A, et al. Changes in bone mass after discontinuation of preexposure prophylaxis with tenofovir disoproxil fumarate/emtricitabine in young men who have sex with men: extension phase results of Adolescent Trials Network protocols 110 and 113. Clin Infect Dis. 2020;70(4):687-91. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7319267/
- Attia S, Egger M, Muller M, et al. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23(11):1397-404. https://journals.lww.com/aidsonline/Fulltext/2009/07170/Sexual_transmission_of_HIV_according_to_viral_load.13.aspx
- Centers for Disease Control and Prevention. Evidence of HIV treatment and viral suppression in preventing the sexual transmission of HIV: technical fact sheet. 2018. Accessed December 1, 2019. https://www.cdc.gov/hiv/risk/art/evidence-of-hiv-treatment.html
- Cohen MS, McCauley M, Gamble TR. HIV treatment as prevention and HPTN 052. Research Support, N.I.H., Extramural Review. Curr Opin HIV AIDS. 2012;7(2):99-105. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3486734/
- Apretude. Prescribing information. ViiV Healthcare; 2021. Accessed January 7, 2022. https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Apretude/pdf/APRETUDE-PI-PIL-IFU.PDF










