Key points
- Clinical laboratories can provide diagnostic testing for pertussis.
- Culture is considered the gold standard.
- Other tests that can be performed include polymerase chain reaction (PCR) and serology.

Test methods
Clinical laboratories commonly use several types of diagnostic tests to identify Bordetella pertussis:
- Culture
- PCR
- Serology
Described below are advantages and disadvantages for each test method.
| Method | Advantages | Disadvantages |
|---|---|---|
| Culture |
|
|
| PCR |
|
|
| Serology |
|
|
Important consideration
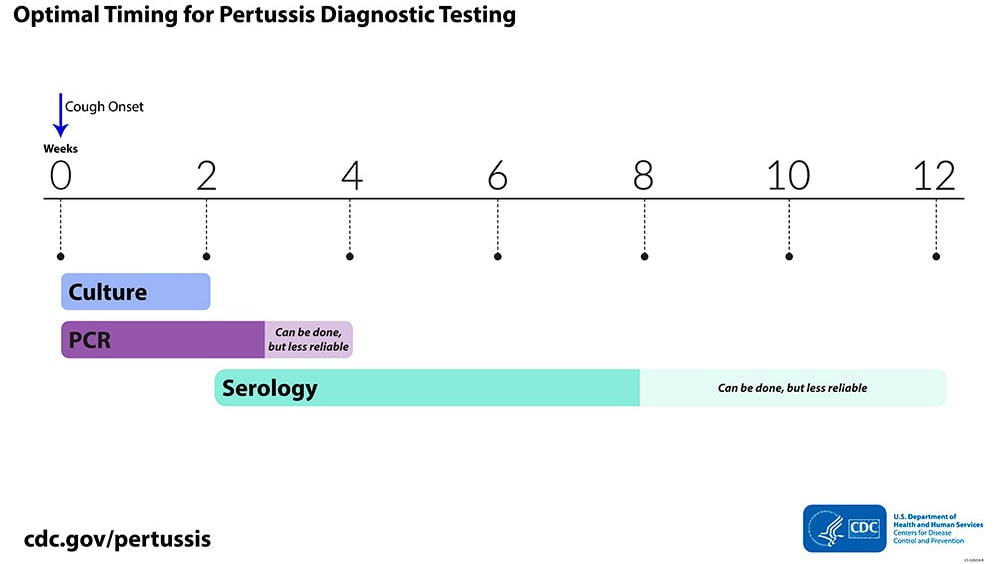
Optimal timing for diagnostic testing
Described below is the optimal timing for when to use each test method.
Culture
- Collect during first 2 weeks of illness following cough onset when viable bacteria are still present
- Sensitivity decreases after the first 2 weeks, which increases risk of false-negative results
PCR
- Use up to 3 to 4 weeks following cough onset
- After fourth week of cough, amount of bacterial DNA in nasopharynx rapidly diminishes, increasing risk of a false-negative result
Serology
- Use 2 to 8 weeks following cough onset for optimal results due to highest antibody titers
- Can be used up to 12 weeks following cough onset
CDC serologic test
CDC and the Food and Drug Administration developed a serologic assay that has been useful for confirming diagnosis, especially during suspected pertussis outbreaks. A few state public health laboratories have included this assay as part of their testing regimen for pertussis. This assay is also available for research or surveillance purposes through CDC's test directory.
CDC evaluation of commercially available assays
Commercially, there are several different serologic tests used in the United States with unproven or unknown clinical accuracy. CDC performed an evaluation of several commercially available assays in the United States and found great variability between them, depending on the antigen or antibody used and whether they were calibrated to an international reference serum. Analysis found the most promising assays were those that measured IgG antibodies against pertussis toxin only and were calibrated to a reference standard.
Specimen collection
Obtaining a nasopharyngeal (NP) swab or aspirate
Healthcare providers should obtain an NP swab or aspirate from everyone with suspected pertussis.
Using specimen for culture
Directly plate collected NP swab or immediately place into transport medium. Plating should occur within 24 hours of NP swab or aspirate collection.
Proper technique for obtaining an NP specimen for isolation
A properly obtained NP swab or aspirate is needed for optimal diagnostic results. The same specimen can be used both for culture and PCR.
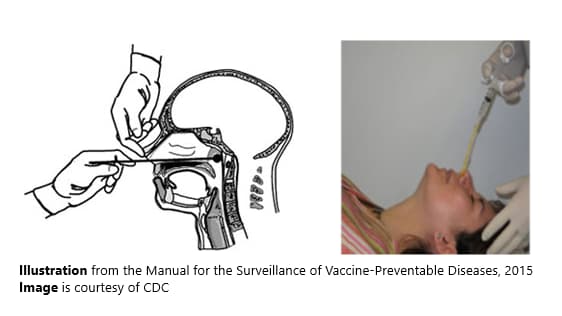
From the Manual for the Surveillance of Vaccine-Preventable Diseases, 2015.
Submitting specimens
Specimen acceptance criteria
CDC only accepts various specimen types for B. pertussis testing from public health laboratories and other federal agencies.
Specimens from private healthcare providers and institutions must be submitted to a public health department laboratory for appropriate processing.
Specimen, documentation, packaging, and shipping
Specimen requirements vary by the specific test requested.
The following links provide information on specimen, documentation, packaging, and shipping requirements:
