At a glance
- Mucormycosis is a rare but severe fungal infection caused by mucormycete molds, which are found throughout the environment.
- Mucormycosis primarily affects people who are immunocompromised and those with uncontrolled diabetes.
- Clinical presentation depends on the body site affected.
- Treatment requires antifungal therapy with amphotericin B, posaconazole, or isavuconazole and often involves aggressive surgery.

Cause
Mucormycosis is caused by molds belonging to the order Mucorales, most commonly Rhizopus species. Other causative species include Mucor species, Cunninghamella bertholletiae, Apophysomyces species, and Lichtheimia (formerly Absidia) species.
Risk factors
People are exposed to mucormycete molds every day without getting sick. Mucormycosis is a risk among people with weakened immune systems. Some medical conditions, blood level abnormalities, medical treatments, and health characteristics or behaviors increase risk for infection.
Medical conditions
- Uncontrolled diabetes.
- Severe COVID-19.
- Malignancy.
Blood level abnormalities
- Persistent neutropenia.
- Iron overload.
Medical treatments
- Stem cell or solid organ transplant.
- Prolonged corticosteroid therapy.
Other patient characteristics
- Trauma, burns, or surgical wounds.
- Intravenous drug use.
- Malnourishment.
- Premature birth.
Mucormycosis and COVID-19
How it spreads
Mucormycosis is acquired through inhalation, inoculation, or ingestion of spores from the environment. Most cases are sporadic, and mucormycosis is not contagious.
Mucormycosis outbreaks are rare. However, there are occasional outbreaks in communities from natural disasters. Flooding and damp conditions promote mold growth and increase injuries.
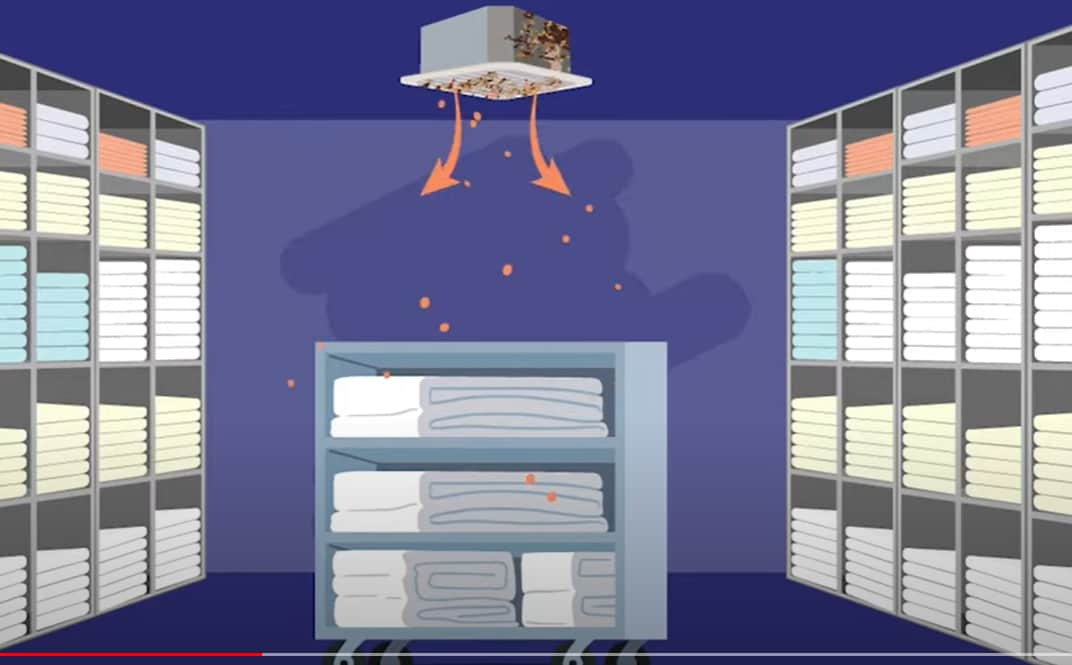
Outbreaks have also occurred in healthcare settings. Sick patients already are much more likely to get mucormycosis. Mold contamination has happened through adhesive bandages, tongue suppressors, water leaks, poor air filtration, dust from nearby construction, and contaminated linens
Clinical features
There are five major clinical forms of mucormycosis; of these, rhinocerebral and pulmonary infections are the most common.
A classic clinical sign of mucormycosis is the rapid onset of tissue necrosis with or without fever. Necrosis is the result of invasion of blood vessels, subsequent thrombosis, and tissue death.
Rhinocerebral mucormycosis is the most common form in patients with diabetes and with renal transplants. It also occurs in neutropenic cancer patients and hematopoietic stem cell transplant or solid organ transplant recipients.
Symptoms may include unilateral facial swelling, headaches, nasal or sinus congestion or pain, serosanguinous nasal discharge, and fever. As the infection spreads, ptosis, proptosis, loss of extraocular muscle function, and vision disturbance may occur. Necrotic black lesions on the hard palate or nasal turbinate and drainage of black pus from eyes are useful diagnostic signs.
Pulmonary mucormycosis generally occurs in patients with hematologic malignancy or profound neutropenia. The symptoms are non-specific and include fever, cough, chest pain, and dyspnea. Angioinvasion results in tissue necrosis, which may ultimately lead to cavitation and/or hemoptysis.
Cutaneous mucormycosis may be primary or secondary. Primary infection is usually caused by direct inoculation of the fungus into disrupted skin. It is most often seen in patients with burns or other forms of local skin trauma, and can occur in patients who are not immunosuppressed.
Primary infection produces an acute inflammatory response with pus, abscess formation, tissue swelling, and necrosis. The lesions may appear red and indurated and often progress to black eschars.
Secondary cutaneous infection is generally seen when the pathogen spreads hematogenously. Lesions typically begin as an erythematous, indurated, and painful cellulitis and then progress to an ulcer covered with a black eschar.
Gastrointestinal mucormycosis is less common than the other clinical forms and is believed to result from ingestion of the organism. It typically occurs in malnourished patients or premature infants.
The stomach, colon, and ileum are most affected. Non-specific abdominal pain and distension, nausea, and vomiting are the most common symptoms, and gastrointestinal bleeding can occur.
It is the most common form of mucormycosis among neonates. It is challenging to diagnose partly because of its clinical resemblance to necrotizing enterocolitis, a far more common disease.
Disseminated mucormycosis but it usually develops from neutropenic patients with a pulmonary infection, but any form of mucormycosis can disseminate. The most common site of spread is the brain, but the spleen, heart, skin, and other organs can also be affected.
Testing

A definitive diagnosis of mucormycosis typically requires histopathological evidence or positive culture from a specimen from the site of infection. Specimens from sterile body sites offer stronger evidence of invasive infection compared to colonization.
Culture of non-sterile sites (e.g., sputum) may be helpful in patients with infection that is clinically consistent with mucormycosis. Mucormycetes may be difficult to differentiate from other filamentous fungi in tissue; experienced pathological and microbiological assistance is often helpful.
No routine serologic tests for mucormycosis are currently available; blood tests such as beta-D-glucan or Aspergillus galactomannan do not detect mucormycetes. DNA-based techniques for detection are promising but are not yet fully standardized or commercially available.
Treatment and recovery
Early recognition, diagnosis, and prompt administration of appropriate antifungal treatment are important for improving outcomes for patients with mucormycosis. Amphotericin B, posaconazole, and isavuconazole are active against most mucormycetes. Lipid formulations of amphotericin B are often used as first-line treatment.
Medications active against Aspergillus such as voriconazole are not active against mucormycetes,. Some evidence to suggest that pre-exposure to voriconazole may be associated with increased incidence of mucormycosis in some patients.
In addition, surgical debridement or resection of infected tissue is often necessary, particularly for rhinocerebral, cutaneous, and gastrointestinal infections. Control of the underlying immunocompromising condition should be attempted when possible. The efficacy of other treatments such as hyperbaric oxygen therapy is uncertain but have been useful in certain situations.
Complications
Patient prognosis depends on several factors. including the rapidity of diagnosis and treatment, the site of infection, and the patient's underlying conditions and degree of immunosuppression. The overall mortality rate is approximately 50%, although early identification and treatment can lead to better outcomes.
- Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012 Feb;54 Suppl 1:S23-34.
- Lewis RE, Kontoyiannis DP. Epidemiology and treatment of mucormycosis. Future Microbiol. 2013 Sep;8(9):1163-75.
- Spellberg B, Edwards Jr. J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005 Jul;18(3):556-69.
- Song Y, Qiao J, Giovanni G, Liu G, Yang H, Wu J, Chen J. Mucormycosis in renal transplant recipients: review of 174 reported cases. BMC Infect Dis. 2017 Apr; 17(1): 283.
- Vallabhaneni S, Mody RK. Gastrointestinal mucormycosis in neonates: a review. Current Fungal Infect Rep. 2015 Sept
- Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005 Sep 1;41(5):634-53.
- Richardson M. The ecology of the Zygomycetes and its impact on environmental exposure. Clin Microbiol Infect. 2009 Oct;15 Suppl 5:2-9.
- Meklin T, Reponen T, McKinstry C, Cho SH, Grinshpun SA, Nevalainen A, et al. Comparison of mold concentrations quantified by MSQPCR in indoor and outdoor airs ampled simultaneously. Sci Total Environ 2007 Sept. 382(1):130–134.
- Calvo MA, Guarro J, Suarez G, Ramirez C. Airborne fungi in the air of Barcelona (Spain). IV. Various isolated genera. Mycopathologia. 1980 Jul 1; 71(2):119–123.
- Klaric MS, Pepeljnjak S. A year-round aeromycological study in Zagreb area, Croatia. Ann Agric Environ Med 2006; 13(1):55–64.
- Ziaee A, Zia M, Bayat M, Hashemi. Identification of Mucorales isolates from soil using morphological and molecular methods. Curr Med Mycol. 2016 Mar;2(1):13-19.
- Rammaert B, Lanternier F, Zahar JR, Dannaoui E, Bougnoux ME, Lecuit M, et al. Healthcare-associated mucormycosis. Clin Infect Dis. 2012 Feb;54 Suppl 1:S44-54.
- Duffy J, Harris J, Gade L, Sehulster L, Newhouse E, O'Connell H, et al. Mucormycosis outbreak associated with hospital linens. Pediatr Infect Dis J. 2014 May;33(5):472-6.
- Novosad SA, Vasquez AM, Nambiar A, Matthew AJ, Christensen E, Moulton-Meissner H, et al. Notes from the field: probable mucormycosis among adult solid organ transplant recipients at an acute care hospital – Pennsylvania, 2014-2015. MMWR Morb Mortal Wkly Rep. 2016;65(18):481-482.
- Garner D, Machin K. Investigation and management of an outbreak of mucormycosis in a paediatric oncology unit. J Hosp Infect. 2008 Sept;70(1):53-59
- Mishra B, Mandal A, Kumar N. Mycotic prosthetic-valve endocarditis. J Hosp Infect. 1992;20(2):122-125.
- Del Palacio Hernanz A, Fereres J, Larregla Garraus S, Rodriguez-Noriega A, Sanz Sanz F. Nosocomial infection by Rhizomucor pusillus in a clinical haematology unit. J Hosp Infect. 1983;4(1):45-49.
- de Repentigny L, St-Germain G, Charest H, Kokta V, Vobecky S. Fatal zygomycosis caused by Mucor indicus in a child with an implantable left ventricular assist device. Pediatr Infect Dis J. 2008;27(4):365-369.
- Chaudhry R, Venugopal P, Chopra P. Prosthetic mitral valve mucormycosis caused by Mucor species. Int J Cardiol. 1987;17(3):333-335.
- Chaves MS, Franco D, Nanni JC, Basaldúa ML, Boleas M, Aphalo G, et al. Control of an outbreak of postoperative bone mucormycosis: an intervention study of contiguous cohorts. Am J Infect Control. 2016;44(12):1715-1717.
- Neblett Fanfair R, Benedict K, Bos J, Bennett SD, Lo YC, Adebanjo T, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. New Engl J Med. 2012 Dec 6;367(23):2214-25.
- Patino JF, Castro D, Valencia A, Morales P. Necrotizing soft tissue lesions after a volcanic cataclysm. World J Surg. 1991 Mar-Apr;15(2):240-7.
- Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT, Kontoyiannis DP. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis. 2012 Feb;54 Suppl 1:S55-60.
- De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008 Jun 15;46(12):1813-21.
- Dadwal SS, Kontoyjannis DP. Recent advances in the molecular diagnosis of mucormycosis. Expert Rev of Mol Diagn. 2018 Oct; 18(10):845-854
- Kontoyiannis P, Lewis RE. How I treat mucormycosis. Blood. 2011;118(5):1216-1224.
- Pongas GN, Lewis RE, 2009. Voriconazole-associated zygomycosis: a significant consequence of evolving antifungal prophylaxis and immunosuppression practices? Clin Microb Infec. 2009 Oct; 15 Suppl 5:93-7.
- John BV, Chamilos G, Kontoyiannis DP. Hyperbaric oxygen as an adjunctive treatment for zygomycosis. Clin Microbiol Infect. 2005 Jul;11(7):515-7.
