Key points
- CDC recommends universal hepatitis C screening for all adults 18 and older and all pregnant people during each pregnancy.
- CDC recommends testing people in certain high-risk groups more frequently.
- Testing, diagnosis, and timely treatment can prevent hepatitis C complications and interrupt transmission.
Why it's important
Nearly one in three people with hepatitis C are unaware of their infection status, and approximately 75%–85% of people with hepatitis C don’t have symptoms.123 Without testing, they can unknowingly transmit the virus to others.
There is no vaccine to prevent hepatitis C. Therefore, the best way to prevent infection is by avoiding behaviors that can transmit the virus.
For the public
How to make decisions on whether to test or screen
Clinicians should universally screen:
- All adults 18 and older at least once in their lifetime, except in settings where the prevalence of hepatitis C virus (HCV) infection (HCV RNA-positivity) is under 0.1%.
- All pregnant people during each pregnancy, except in settings where the prevalence of HCV infection (HCV RNA-positivity) is under 0.1%.
CDC recommends one-time hepatitis C testing for people with recognized risk factors or exposures, including:
- People who currently or have previously injected drugs and shared needles, syringes, or other drug preparation equipment.
- People with human immunodeficiency virus (HIV).
- People with selected medical conditions, including people who have ever received maintenance hemodialysis and persons with persistently abnormal alanine aminotransferase (ALT) levels.
- Prior recipients of transfusions or organ transplants, including:
- People who received clotting factor concentrates produced before 1987.
- People who received a transfusion of blood or blood components before July 1992.
- People who received an organ transplant before July 1992.
- People who were notified that they received blood from a donor who later tested positive for HCV infection.
- People who received clotting factor concentrates produced before 1987.
- Health care, emergency medical, and public safety personnel after needle sticks, sharps, or mucosal exposures to HCV-positive blood.
- Infants born to people with known hepatitis C.
CDC also recommends routine periodic testing for patients with ongoing risk factors (regardless of setting prevalence), including:
- People who currently inject drugs and share needles, syringes, or other drug preparation equipment.
- People with selected medical conditions, including people who receive maintenance hemodialysis.
Clinicians should test anyone who requests a hepatitis C test, regardless of stated risk factors, because patients may be hesitant to share stigmatizing risks.
Screening and testing guidelines
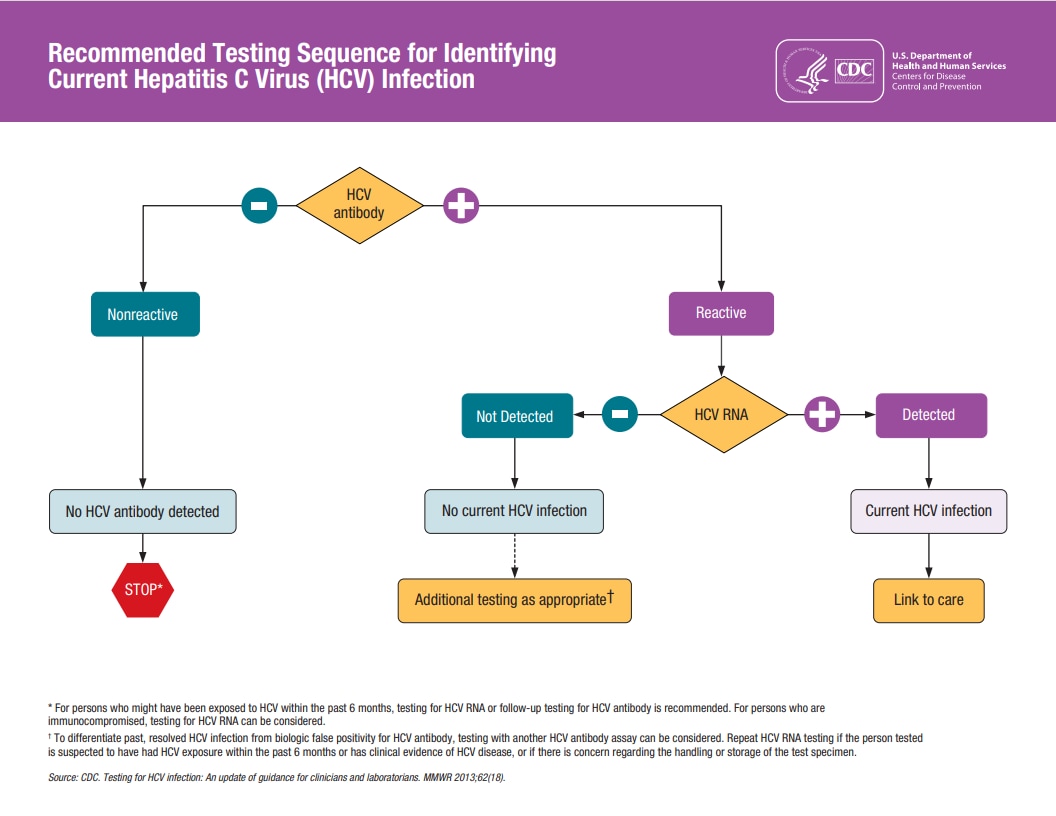
Clinicians should initiate hepatitis C testing with an HCV antibody test with reflex to NAT for HCV RNA if the antibody test is positive/reactive. See the complete testing sequence.
If a patient’s antibody test is positive/reactive and they also have detectable HCV RNA, they have a current HCV infection and you should counsel the patient, evaluate for treatment, and initiate an appropriate direct-acting antiviral (DAA) regimen.
Operational guidance for complete hepatitis C testing
It is important to reduce time to diagnosis, evaluation, and treatment initiation. CDC recommends that clinicians collect all samples needed to diagnose hepatitis C in a single visit and order HCV RNA testing automatically when the HCV antibody is reactive.4 When the HCV antibody test is reactive, the laboratories should automatically perform NAT testing for HCV RNA detection. This automatic testing streamlines the process because it occurs without any additional action on the part of the patient or the clinician. See complete recommendations.
Possibility of false negatives
During acute HCV infection, antibody becomes detectable at 8–11 weeks. Therefore, a patient who thinks they may have been recently exposed might not have antibody levels high enough for a positive HCV antibody test. In addition, some people might lack the immune response necessary to develop detectable antibodies within this time range. In cases like these, clinicians should consider virologic testing.56
HCV RNA testing for recent infection
HCV RNA testing is recommended for the diagnosis of current HCV infection among people who might have been exposed to HCV within the past 6 months, regardless of HCV antibody result. HCV RNA becomes detectable approximately 1 – 2 weeks after infection with HCV. Suspected exposure may be inferred from the patient's history or the context and setting of the patient encounter (e.g., inferred potential exposure among people who inject drugs presenting to a syringe service program).
Implementation Considerations
Both laboratory and POC tests for HCV antibody and HCV RNA are currently available. There are several considerations to review when choosing a testing strategy. This document, Considerations for the Implementation of Point-of-Care Testing for the Diagnosis of Hepatitis C Virus Infection, details the various considerations for what testing approach to use, where to implement different modalities of testing, and what strategies to use to pair HCV screening and testing with highly accessible treatment.
Recommended tests
Clinicians should use an FDA-approved HCV antibody test followed by a NAT for HCV RNA test when antibody is positive/reactive. Tests include:
- HCV antibody test (anti-HCV) (e.g., enzyme immunoassay [EIA]).
- Nucleic acid test (NAT) to detect presence of HCV RNA (qualitative RNA test).
- NAT to detect levels of HCV RNA (quantitative RNA test).
A reactive HCV antibody test result indicates a history of past or current HCV infection. A detectable HCV RNA test result indicates current infection.
NAT for detection of HCV RNA should be used among people with suspected HCV exposure within the past 6 months.
Clinicians should test all perinatally exposed infants for HCV RNA using a NAT at 2–6 months. Care for infants with detectable HCV RNA should be coordinated in consultation with a provider who has expertise in pediatric hepatitis C management. Infants with undetectable HCV RNA do not require further follow-up unless clinically warranted.
How to interpret screening results
Interpretation of Results of Tests for HCV Infection and Further Actions
Test Outcome
Interpretation
Further Actions
HCV antibody nonreactive
No HCV antibody detected
Sample can be reported as nonreactive for HCV antibody. No further action required. If recent exposure is suspected, test for HCV RNA.*
HCV antibody reactive
Presumptive HCV infection
A repeatedly reactive result could be consistent with current HCV infection, past HCV infection that has resolved, or biologic false positivity for HCV antibody. Test for HCV RNA to identify current infection.
HCV antibody reactive, HCV RNA detected
Current HCV infection
Provide person tested with appropriate counseling and link to care and treatment.†
HCV antibody reactive, HCV RNA not detected
No current HCV infection
No further action required in most cases. If distinction between true positivity and biologic false positivity for HCV antibody is desired, and if sample is repeatedly reactive in the initial test, test with another HCV antibody assay. In certain situations, follow up with HCV RNA testing and appropriate counseling.§
* If HCV RNA testing is not feasible and person tested is not immunocompromised, do follow-up testing for HCV antibody to demonstrate seroconversion. If the patient tested is immunocompromised, consider testing for HCV RNA.
† It is recommended before initiating antiviral therapy to retest for HCV RNA in a subsequent blood sample to confirm HCV RNA positivity.
§ If the patient is suspected of having HCV exposure within the past 6 months, or has clinical evidence of HCV disease, or if there is concern regarding the handling or storage of the test specimen, the clinician should follow up with the patient.
Traditional diagnostic sequence flow chart
Other resources:
How to diagnose Hepatitis C
If an HCV antibody test is not reactive/positive, the patient has not been exposed and you can rule out infection.
For acute HCV infection, it usually takes 8–11 weeks before antibodies are detectable.
If the antibody test is reactive/positive, you will still need to test for HCV RNA to diagnose the patient and start treatment.
It usually takes 1–2 weeks after exposure to the virus for detectable HCV RNA levels to appear.7
New guidance from CDC in July 2023 recommends complete, automatic HCV RNA testing on all HCV antibody reactive samples to minimize patient visits and increase the number of patients diagnosed and treated.
What to do next
CDC recommends that clinicians offer the following services to people who are diagnosed with HCV infection:
- Medical evaluation (by either a primary care clinician or specialist for chronic liver disease, including treatment and monitoring).
- Hepatitis A and hepatitis B vaccination.
- Screening and brief intervention for alcohol consumption.
- HIV risk assessment and testing.
More information on recommendations for testing, management, and treating hepatitis C are available from CDC and the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA).
Reporting cases
The National Notifiable Diseases Surveillance System (NNDSS) lists acute, chronic, and perinatal hepatitis C as nationally notifiable conditions.8
You should report cases of suspected health care-associated HCV infection to state and local public health authorities for prompt investigation and response.
When you report a case, you will use an event code that corresponds to the hepatitis C condition. You can reclassify cases if needed, as long as the changes occur before surveillance data are finalized each year.
National Event codes are:
- 10101
- 10105
- 5024
Resources
- Updated Operational Guidance for Implementing CDC's Recommendations on Testing for Hepatitis C Virus Infection | MMWR
- CDC Recommendations for Hepatitis C Testing Among Perinatally Exposed Infants and Children — United States, 2023 | MMWR
- CDC Recommendations for Hepatitis C Screening Among Adults | MMWR
- Recommendations for Testing, Managing, and Treating Hepatitis C | HCV Guidance
- Interpretation of Test Results for Hepatitis C Virus (HCV) Infection (Graph)
- Recommended HCV Testing Sequence Flow Chart (Flow Chart)
- Algorithm for Health Care Personnel Exposed to HCV (Flow Chart)
- Updated HCV Testing Guidance for Clinicians and Laboratorians | MMWR
- Lewis KC, Barker LK, Jiles RB, Gupta N. Estimated Prevalence and Awareness of Hepatitis C Virus Infection Among US Adults: National Health and Nutrition Examination Survey, January 2017-March 2020. Clin Infect Dis. 2023 Nov 17;77(10):1413-1415.
- Maheshwari A, Ray S, Thuluvath PJ. Acute hepatitis C. Lancet 2008;372:321-332.
- Grebely J, Matthews GV, Dore GJ. Treatment of acute HCV infection. Nat Rev Gastroenterol Hepatol 2011;8:265–274.
- Cartwright EJ, Patel P, Kamili S, Wester C. Updated Operational Guidance for Implementing CDC's Recommendations on Testing for Hepatitis C Virus Infection. MMWR Morb Mortal Wkly Rep 2023;72:766–768. DOI: http://dx.doi.org/10.15585/mmwr.mm7228a2.
- Thomson EC, et al. Delayed anti-HCV antibody response in HIV-positive men acutely infected with HCV. AIDS, 2009. 23(1): p. 89-93.
- Vanhommerig, JW, et al. Hepatitis C virus (HCV) antibody dynamics following acute HCV infection and reinfection among HIV-infected men who have sex with men. Clin Infect Dis, 2014. 59(12): p. 1678-85.
- Association of Public Health Laboratories (APHL). Infectious Diseases, January 2019. Interpretation of Hepatitis C Virus Test Results: Guidance for Laboratories [cited 2019 June 25]; Available at: https://www.aphl.org/aboutAPHL/publications/Documents/ID-2019Jan-HCV-Test-Result-Interpretation-Guide.pdf.
- Centers for Disease Control and Prevention: Lesson 5: Public health surveillance. Appendix A. Characteristics of well-conducted surveillance. In: Dicker RC, Coronado F, Koo D, Parrish RG, eds. Principles of Epidemiology in Public Health Practice, Third Edition: An Introduction to Applied Epidemiology and Biostatistics. Atlanta, Georgia, 2006.
- Centers for Disease Control and Prevention (CDC). Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013 May 10;62(18):362-5. PMID: 23657112; PMCID: PMC4605020.
