Key points
- The results of a blood tests will determine if a person is infected with the hepatitis A virus (HAV).
- Diagnosis and prompt administration of postexposure prophylaxis (PEP) to potentially exposed contacts can interrupt transmission.
- Clinicians should report positive cases to local health authorities.
Why it's important
It's critical to identify hepatitis A cases early to prevent symptomatic illness and transmission. Administering PEP to contacts of patients diagnosed with hepatitis A within 2 weeks of exposure is highly effective in preventing HAV infection.
In the United States, person-to-person transmission can lead to increased infection rates, especially among people who are at high risk for HAV infection, such as:
- People who use or inject drugs.
- People experiencing homelessness.
- Men who have sex with men.
How to make decisions on whether to test or screen
A blood test can confirm a suspected case of hepatitis A. The types of diagnostic tests used to confirm an HAV infection include serologic testing and, occasionally, PCR-based tests.
Prevaccination testing
In most instances, it’s not routinely recommended to administer serologic testing for hepatitis A before vaccination. However, you may consider it in specific instances when the cost of vaccinating people who are already immune is a concern.
Screening and testing guidelines
You will not be able to differentiate hepatitis A virus from other types of viral hepatitis using clinical or epidemiological features alone. Clinicians should conduct test(s) to make an accurate diagnosis.
The following are laboratory markers that, if present, indicate an acute HAV infection:
- Immunoglobulin M antibodies to HAV (IgM anti-HAV) in serum, or
- HAV RNA is serum or stool1
The following is the serologic marker that, if present, indicates either immunity from prior infection or vaccination:
- Immunoglobulin G antibodies to HAV (IgG anti-HAV)
Serologic tests for IgG anti-HAV and total anti-HAV (IgM and IgG anti-HAV combined) are not helpful in diagnosing acute illness. You should only test patients for IgM anti-HAV if they are symptomatic, and you suspect HAV infection. Alanine aminotransferase (ALT) and total bilirubin tests can aid in diagnosis.
How to interpret test results
This table can help you interpret hepatitis A laboratory results.
Total anti-HAV
Anti-HAV IgM
Interpretation
Positive
Positive
Current infection, recent infection, or recent vaccination.
Positive
Not done
Previous infection or current infection; cannot differentiate recent from remote infection or prior vaccination.
Positive
Negative
Previous infection or vaccination.
Negative
Negative
Not infected (i.e., susceptible).
Not done or negative
Positive
Current infection or false-positivity/cross-reactivity.
Serological course of hepatitis A
CDC offers an online training that covers hepatitis A serology.
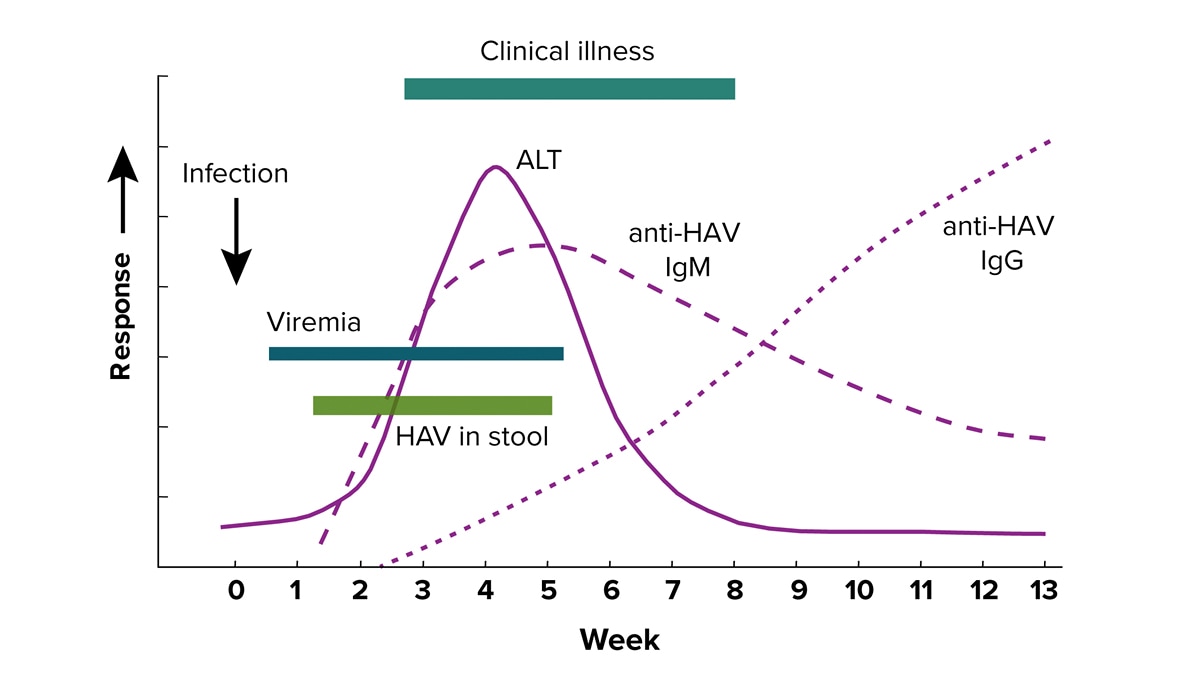
IgM anti-HAV is typically present from 5-10 days before the onset of clinical symptoms to 6 months after infection. IgG anti-HAV typically becomes detectable at or right before the onset of clinical illness and persists to provide lifelong immunity.
How to diagnose hepatitis A
Clinicians should assess a patient's history (including potential risk behaviors or exposures for HAV infection), physical exam, and test results in determining whether hepatitis A is the appropriate clinical diagnosis for a patient. The Council of State and Territorial Epidemiologists developed a surveillance case definition for acute hepatitis A. This set of uniform criteria is used to define hepatitis A for public health surveillance and can be found here: Hepatitis A, Acute 2019 Case Definition | CDC.
Learn more about viral hepatitis serology.
What to do next
Most of the time, rest and a balanced diet with healthy food and plenty of fluids are enough to treat hepatitis A symptoms. In some cases, people with severe symptoms will need medical care in a hospital.
Learn more about treating hepatitis A.
Reporting cases
Clinicians and health care providers should report cases of hepatitis A to health departments as specified by state, territorial, and local regulations.
CDC shares annual surveillance reports with data on national hepatitis A occurrences. Some cases may be reclassified or removed before the data are finalized each year.
- Centers for Disease Control and Prevention (CDC) Online Viral Hepatitis Serology Training | CDC Atlanta, GA. US Department of Health and Human Services, 2015.
