What to know
Examples of institutional or semi-closed settings include long-term care facilities, nursing homes, schools, correctional facilities, hospitals, dormitories, and cruise ships.
Guide to use of influenza virus diagnostic tests1
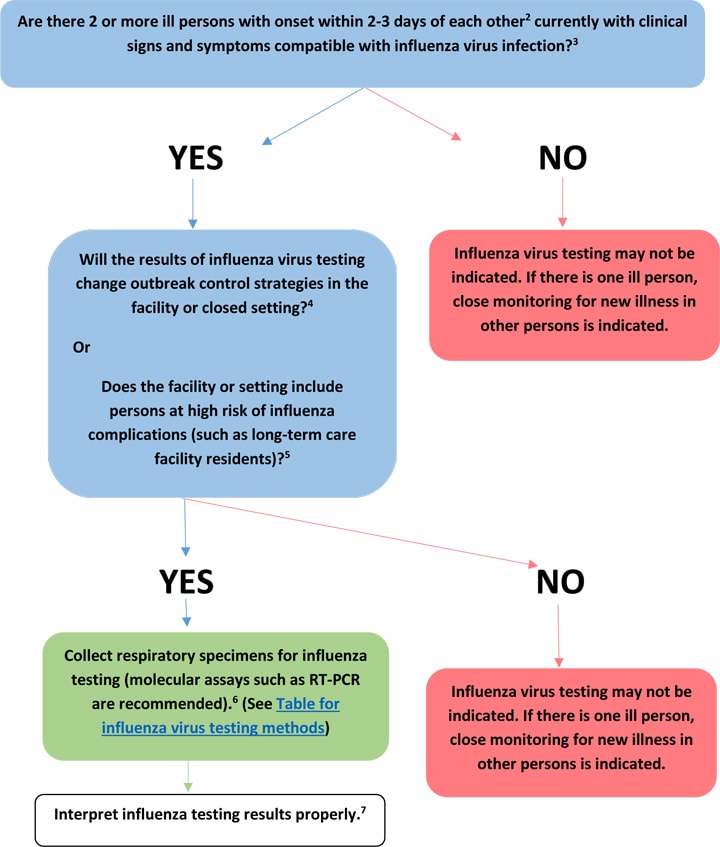
- Examples of institutional or semi-closed settings include long-term care facilities, nursing homes, schools, correctional facilities, hospitals, dormitories, and cruise ships.
- In settings with persons at high-risk of influenza complications, a single case of suspected influenza is sufficient for triggering influenza testing and prompt implementation of infection prevention and control measures, including active surveillance for new illness cases. The Infectious Diseases Society of America (IDSA) Influenza Clinical Practice Guidelines recommends implementation of outbreak control measures in a long-term care facility when 2 cases of healthcare associated laboratory-confirmed influenza are identified within 72 hours of each other in residents or patients of the same ward or unit. Interim Guidance for Influenza Outbreak Management in Long-Term Care and Post-Acute Care Facilities
- The signs and symptoms of influenza can vary by age, immune status, and presence of underlying medical conditions. Persons with uncomplicated influenza may be afebrile or present with Influenza like-illness (fever with either cough or sore throat), fever with other respiratory symptoms. Other symptoms associated with influenza include myalgias, headache, and fatigue. Note that some persons may have atypical presentations (e.g., older adults, very young infants, immunosuppressed, and patients with certain chronic medical conditions). Complications include exacerbation of underlying chronic disease, (e.g., congestive cardiac failure, asthma), pneumonia, secondary bacterial pneumonia, bronchiolitis, croup, encephalopathy, encephalitis, seizures, myocarditis, myositis, and others.
- For example, influenza test results may help to guide decisions about whether empiric antiviral treatment of ill persons should be continued for all patients or only those with confirmed influenza virus infection; whether post-exposure chemoprophylaxis should be initiated in persons with exposure to a person with confirmed influenza virus infection; and for guiding infection prevention and control practices (isolation or cohorting of ill persons, quarantining of exposed persons, changes in admission or staffing policies, or changes in social distancing recommendations, etc). Influenza Antiviral Medications: Summary for Clinicians and Infection Control in Health Care Facilities has more information.
- Persons at higher risk for developing influenza-related complications include those aged ≥65 years or <2 years; pregnant women; persons with chronic lung disease (including asthma), heart disease, renal, metabolic, hematologic and neurologic disease; immunosuppression; and morbid obesity. People at High Risk of Developing Flu-Related Complications has more information.
- In an outbreak setting, RT-PCR or other molecular assays are preferred. Because of the low to moderate sensitivity of antigen detection assays such as rapid influenza diagnostic tests (RIDTs) or immunofluorescence assays, false negative results are possible and testing of specimens from more than one ill person is recommended because use of RIDTs or immunofluorescence assays may miss detection of an influenza outbreak. The presence of any influenza positives among persons with clinically compatible illnesses suggests that influenza is a probable cause of the outbreak. Negative RIDT results do not exclude influenza virus infection due to low sensitivity to detect influenza viruses. Confirmation of RIDT results by more specific influenza testing (RT-PCR and viral culture) is indicated. Table 1: Overview of Influenza Testing Methods has more information.
- Proper interpretation of influenza testing results is very important. Figure: Influenza testing algorithm to help interpret results when influenza viruses ARE circulating in the community and Figure: Influenza testing algorithm to help interpret results when influenza viruses are NOT circulating in the community is available. The Infectious Diseases Society of America (IDSA) Influenza Clinical Practice Guidelines provides information on interpretation of influenza testing results.
