Key points
- A dengue vaccine provides your child with safe, effective, and long-lasting protection against dengue illness, hospitalization, and severe disease.
- Children need to be tested to confirm a previous dengue infection before vaccination.
- Three doses of the vaccine are required for full protection.
- Talk to your child's healthcare provider to find out if your child is eligible for a dengue vaccine.

Notice about Dengvaxia
Sanofi-Pasteur will stop manufacturing its dengue vaccine for children. The manufacturer is discontinuing the vaccine citing a lack of demand in the global market to continue production of this vaccine. CDC, in collaboration with the Puerto Rico Department of Health, will continue alerting health professionals about the discontinuation of Dengvaxia and the use of this vaccine as recommended by the Advisory Committee on Immunization Practices (ACIP). Dengvaxia is safe and effective when administered as recommended. There are two other dengue vaccines either approved or in late stages of development. However, they are not currently available in the United States. People can continue to protect themselves and their families from dengue by preventing mosquito bites and controlling mosquitoes in and around their homes.
Overview
- Dengvaxia is the only dengue vaccine currently available in the United States.
- Dengvaxia is recommended to prevent dengue in children aged 9–16 years, with laboratory-confirmed previous dengue virus infection and living in areas where dengue is common.
- Dengue is common in the U.S. territories of American Samoa, Puerto Rico, and the U.S. Virgin Islands, and the freely associated states, including the Federated States of Micronesia, the Republic of Marshall Islands, and the Republic of Palau.
- Dengue is common in the U.S. territories of American Samoa, Puerto Rico, and the U.S. Virgin Islands, and the freely associated states, including the Federated States of Micronesia, the Republic of Marshall Islands, and the Republic of Palau.
Test before vaccination
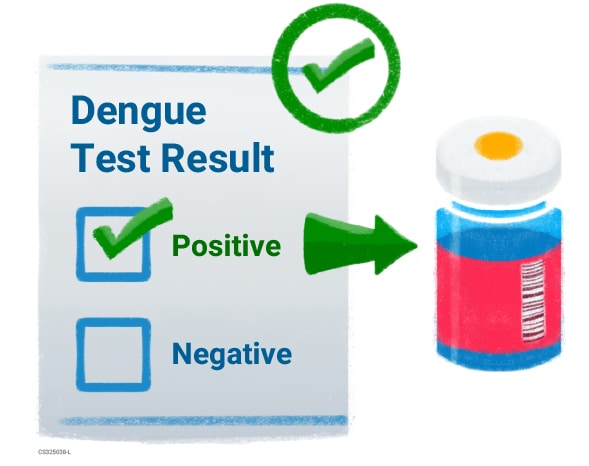
- Laboratory confirmation of a previous dengue infection is required for vaccination with Dengvaxia.
- Testing before vaccination will confirm that your child has had dengue before.
- In children who have not already had dengue, the dengue vaccine increases the risk of hospitalization and severe illness if the child gets dengue after vaccination.
- To reduce the risk of vaccinating children who have never had dengue, a blood test can check for a previous dengue infection.
- If the test result is positive, your child can be vaccinated.
- If the test result is negative, your child won't be able to get vaccinated. Your child should be tested again in one or two years.
- If the test result is positive, your child can be vaccinated.
Types of testing
Your child's healthcare provider will order a blood test to confirm a previous dengue infection. The healthcare provider will order a test that has been approved by the local or territorial health department.
OR
If your child had a previous dengue illness that was confirmed by a specific type of laboratory test, your child may not need an additional blood test.
Parents:
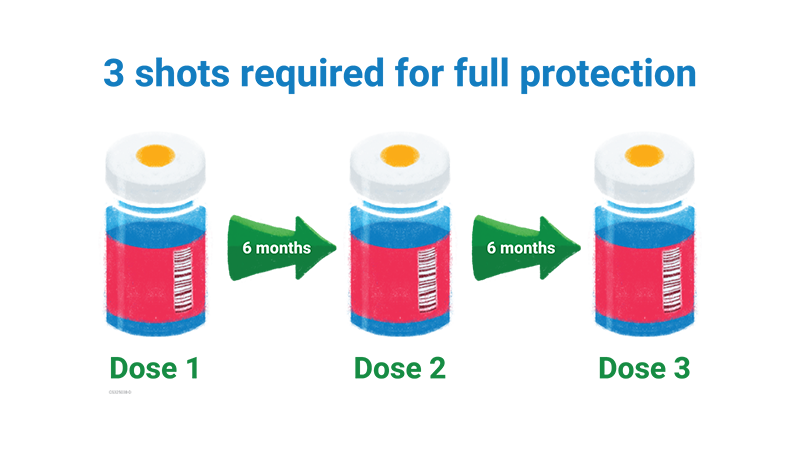
Three doses required
- Dose 1 can be given after confirming that your child had a prior dengue infection.
- Dose 2 is given 6 months after the first dose.
- Dose 3 is given 6 months after the second dose.
Safety & efficacy
- The dengue vaccine protects against all four types of dengue.
- The vaccine is safe and effective among children who have previously had dengue.
- The vaccine has few side effects.
- The most common side effects are soreness, itchiness, or pain in the injection site, headaches, lack of energy, and general discomfort.
- These side effects are normal signs that the body is building protection, and the side effects should go away within a few days.
- The most common side effects are soreness, itchiness, or pain in the injection site, headaches, lack of energy, and general discomfort.

Special populations
Pregnancy
Pregnant people were not specifically enrolled and studied in the vaccine trials. Although no significant differences in adverse pregnancy outcomes between vaccinated and unvaccinated people were found, the number of pregnant people enrolled was too small to determine the effect of Dengvaxia on pregnancy.
Lactation
No data are available to evaluate Dengvaxia and breastfeeding.
