Key points
- One chikungunya vaccine (manufactured by Valneva and called IXCHIQ) is available in the United States.
- The vaccine is licensed for adults aged 18 years and older.
- The vaccine should be considered for some travelers at higher risk of exposure to chikungunya virus or at increased risk of severe disease.

Chikungunya vaccine
A live attenuated chikungunya vaccine (manufactured by Valneva as IXCHIQ) is the only chikungunya vaccine currently licensed in the United States. This vaccine was approved by the Food and Drug Administration (FDA) in November 2023. The U.S. Advisory Committee on Immunization Practices (ACIP) approved recommendations for use of the vaccine in travelers and laboratory workers in February 2024.
Vaccine administration, contraindications, and precautions
IXCHIQ is licensed for use in adults aged 18 years and older. It is administered intramuscularly as a single 0.5mL dose. There are currently no recommendations for a booster dose of vaccine.
To minimize the risk of serious adverse events, healthcare providers should carefully observe the contraindications and consider the precautions about vaccination prior to vaccine administration.
Contraindications
- Immunocompromising condition (due to immunodeficiencies or immunosuppressive and immunomodulatory therapies)
- History of a severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine.
Precautions
- Pregnancy
- Pregnant persons should avoid the risk for chikungunya virus infection, if possible.
- In general, vaccination should be deferred until after delivery. However, when the risk of infection is high and exposure cannot be avoided, a health care provider should discuss with a pregnant person the potential risks of chikungunya virus infection and the potential benefits and risks of vaccination so that vaccination can be considered.
- If pregnant persons choose to be vaccinated, out of caution vaccination should generally be avoided during the 1st trimester (until 14 weeks gestation) and after the 36th week of gestation.
- Pregnant persons should avoid the risk for chikungunya virus infection, if possible.
- Breastfeeding
- Breastfeeding persons and their infants should avoid the risk for chikungunya virus infection, if possible.
- In the absence of data, breastfeeding is a precaution for vaccination. However, when the risk of infection is high (e.g., during an outbreak) and exposure cannot be avoided, a health care provider should discuss with a breastfeeding person the health benefits of breastfeeding for the infant, the risks of chikungunya virus infection, and the potential benefits and risks of vaccination, and offer the vaccine to the breastfeeding person.
- Breastfeeding persons and their infants should avoid the risk for chikungunya virus infection, if possible.
Vaccine immunogenicity and side effects
Clinical trial results indicated that vaccination resulted in seroresponse rates ≥96% through at least 6 months after vaccination. Data are being gathered on responses over the longer term.
Common adverse reactions following vaccination that occurred in >10% of vaccinated persons in clinical trials included tenderness, headache, fatigue, myalgia, arthralgia, fever, and nausea. IXCHIQ also caused severe or prolonged chikungunya-like adverse reactions in some persons.
More information about IXCHIQ can be found in the IXCHIQ product information available at the FDA IXCHIQ web page.
Healthcare providers are encouraged to report all adverse events that might be caused by vaccination to the CDC/FDA Vaccine Adverse Events Reporting System (VAERS) by submitting a report online or by using a PDF Form
Considerations for chikungunya vaccine for travelers
All travelers to countries or territories with risk of chikungunya virus transmission should take steps to avoid mosquito bites. The risk for chikungunya for most U.S. travelers is low. However, some travelers are at increased risk for infection or more severe disease. Factors to assess when considering use of chikungunya vaccine include the likelihood of exposure to chikungunya virus, a traveler's risk factors for severe disease outcomes, and traveler preferences.

Factors to assess when considering chikungunya vaccine
Chikungunya vaccine recommendations for travelers
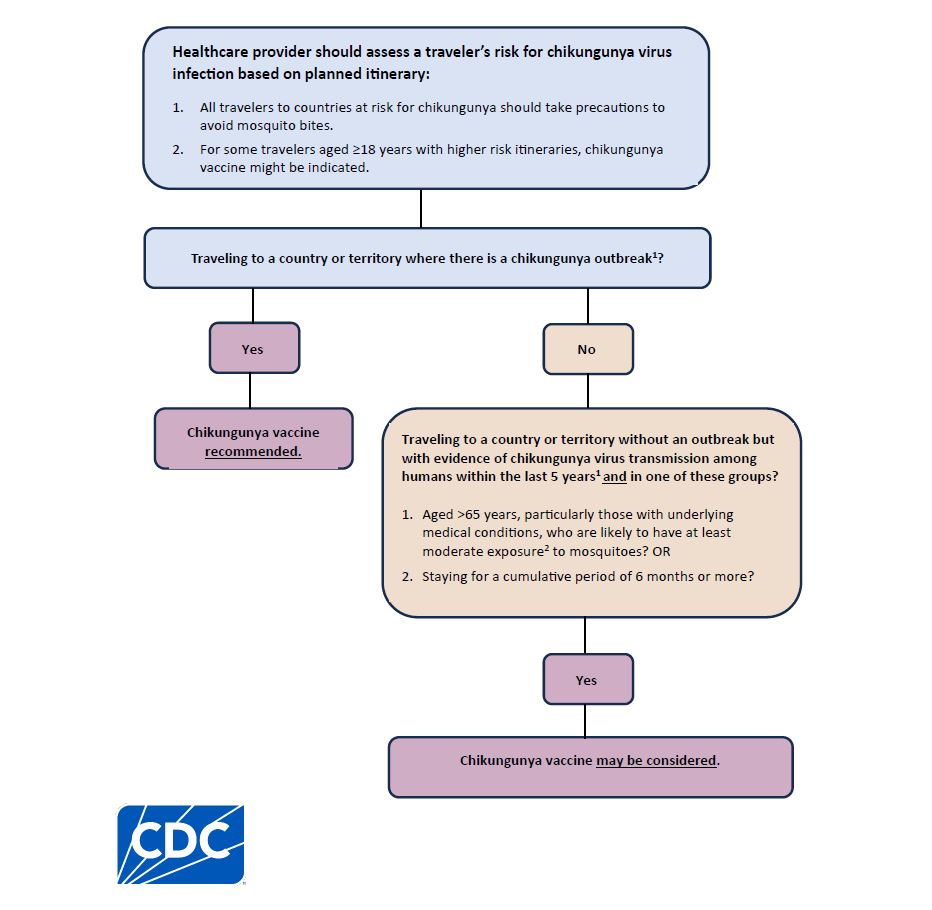
Chikungunya vaccine is recommended for persons aged 18 years and older traveling to a country or territory where there is a chikungunya outbreak.
Chikungunya vaccine also may be considered for the following persons traveling to a country or territory without an outbreak but with evidence of chikungunya virus transmission among humans within the last 5 years:
- Persons aged >65 years, particularly those with underlying medical conditions, who are likely to have at least moderate exposure* to mosquitoes, OR
- Persons staying for a cumulative period of 6 months or more.
*Moderate exposure could include travelers who might have at least 2 weeks (cumulative) of exposure to mosquitoes in indoor or outdoor settings. It does not include travelers who might have limited exposure to mosquitoes (e.g., business travelers likely to be mainly in mosquito-protected indoor settings).
Healthcare providers can use CDC's decision tree to help determine when chikungunya vaccination for travelers is appropriate.
Chikungunya vaccination for U.S. travelers decision tree
Chikungunya vaccination for laboratory workers
Chikungunya virus transmission through the aerosol and percutaneous routes has been documented in the laboratory setting, and transmission through accidental mucosal exposure is possible. Laboratory work with chikungunya virus is generally restricted to biosafety level-3 (BSL-3) facilities and practices.
Chikungunya vaccine is recommended for laboratory workers with potential for exposure to chikungunya virus.
A local institutional biosafety committee should undertake a risk assessment of the potential for exposure to chikungunya virus for each laboratory worker working with the virus. The committee should consider the type of work to be performed and the biosafety level at which work will be conducted. Vaccination is not necessary for workers handling clinical samples who should routinely use standard practices for handling patient samples.
