At a glance
This CDC report analyzes the impact of the COVID-19 pandemic on adolescent vaccination rates of ages 12-13 and provides information on vaccination coverage for ages 14-17.
Background and Purpose
The Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination of adolescents aged 11–12 years with tetanus, diphtheria, and acellular pertussis vaccine (Tdap), human papillomavirus (HPV) vaccine, and meningococcal serogroups A, C, W, Y vaccine (MenACWY)1. On September 2, 2022, CDC released a report using data from the National Immunization Survey-Teen (NIS-Teen) which analyzed the potential effect of the COVID-19 pandemic on routine adolescent vaccination by ages 12 and 13 years among adolescents born 2006-20082. This report provides additional information on potential effect of the pandemic on adolescent vaccination coverage by ages 14 through 17 years.
Methods
To estimate vaccination coverage, CDC analyzed data for 18,002 adolescents aged 13–17 years from the 2021 National Immunization Survey-Teen (NIS-Teen). Vaccination coverage estimates are based on provider-reported vaccination histories and include any vaccines administered before the 2021 NIS-Teen interview date. The cumulative percent of adolescents vaccinated by age in years (12 to 17 years) was assessed, stratified by year of birth (2002-2008). To assess vaccination coverage by ages 14 to 17 years, Kaplan-Meier estimation was used to account for censoring of vaccination status at ages 14 and older. To assess potential COVID-19 pandemic effects for ≥1 HPV vaccine, ≥1 MenACWY, and ≥1 Tdap, vaccination coverage by age 12 years was compared for adolescents born in 2008 (reached age 12 years in 2020, during the pandemic) to those born in 2007 (reached age 12 years in 2019, pre-pandemic); vaccination coverage by age 13 years was compared for adolescents born in 2007 and 2008 (reached age 13 years in 2020 and 2021) to those born in 2006 (reached age 13 years in 2019). Reference birth years for vaccination coverage difference estimates by ages 14, 15, 16, and 17 years were the 2005, 2004, 2003, and 2002 birth years, respectively. Children in these reference birth years reached the given age in 2019 (e.g., for coverage by age 14 years, children born in 2005 reached 14 in 2019, which was pre-pandemic). Analyses were conducted using SAS-Callable SUDAAN (version 11; RTI International). This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.
Results
Coverage with ≥1 HPV vaccine dose among adolescents who reached 12, 13, 14, 15, 16 or 17 years of age during the pandemic (in 2020 or 2021) was similar to coverage before the pandemic.
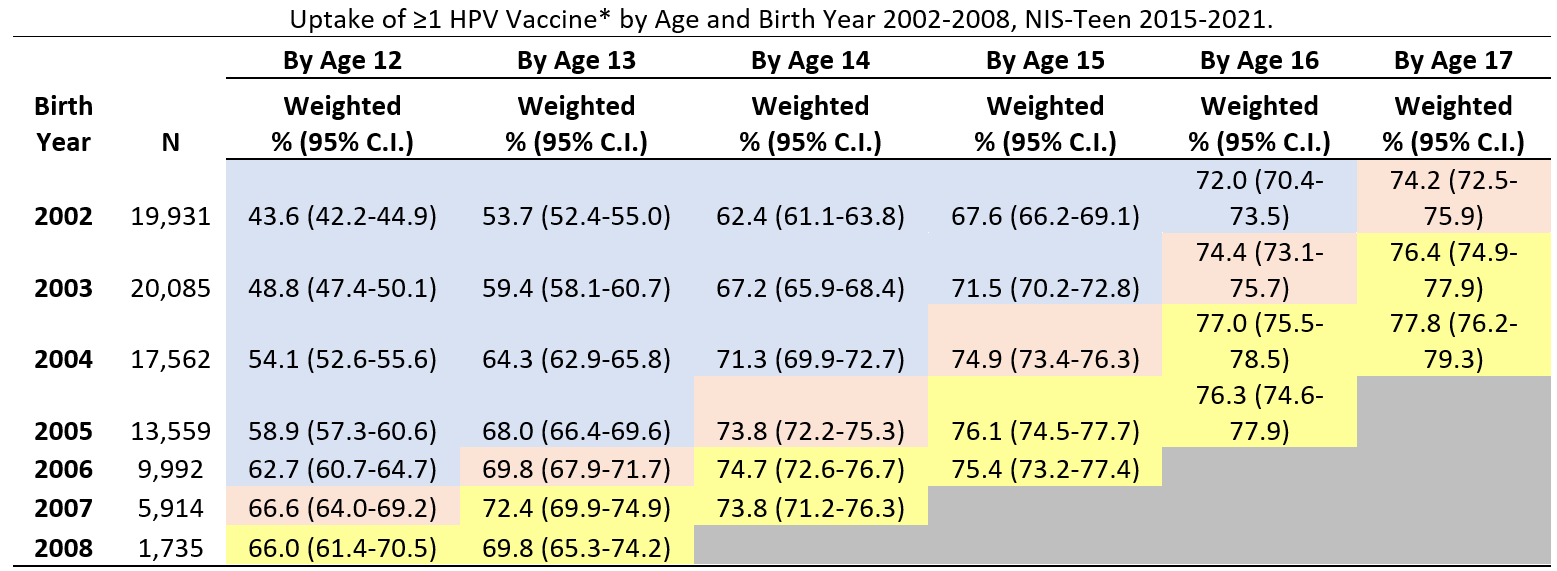
Abbreviations: HPV = human papillomavirus; CI = confidence interval; NIS = National Immunization Survey.
* HPV vaccine includes nine-valent (9vHPV), quadrivalent (4vHPV), or bivalent (2vHPV) in females and males combined.
† Table cells are shaded according to whether persons with the indicated birth year (rows) reached the age indicated (columns) in 2019 or earlier (pre-pandemic, shaded in blue or pink) or in 2020 or 2021 (during pandemic, shaded in yellow). Cells shaded in pink were used as the pre-pandemic referent cell for comparisons with pandemic cells, for each age indicated (column).
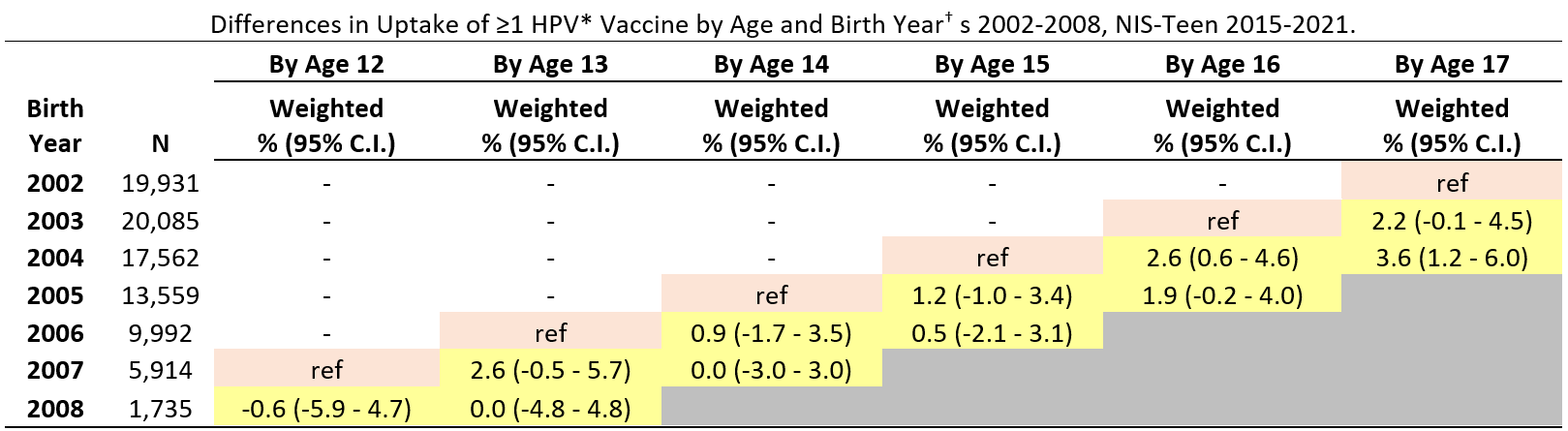
Abbreviations: HPV = human papillomavirus; CI = confidence interval; NIS = National Immunization Survey.
* HPV vaccine includes nine-valent (9vHPV), quadrivalent (4vHPV), or bivalent (2vHPV) in females and males combined.
† Reference birth years (denoted in pink cell shading) for vaccination coverage difference estimates by ages 14, 15, 16, and 17 years were the 2005, 2004, 2003, and 2002 birth years, respectively. Children in these reference birth years reached the given age in 2019 (pre-pandemic year) (e.g., for coverage by age 13 years, children born in 2006 reached 13 in 2019, which was before the pandemic). The reference pre-pandemic year (pink cell shading) is compared to pandemic years (2020 and 2021, yellow cell shading)
Coverage with ≥1 Tdap dose was 4.1 percent points lower (95% confidence interval, -8.1, -0.1) among adolescents who reached 12 during the pandemic (in 2020) than among those who reached 12 before the pandemic (in 2019). Tdap coverage among adolescents who reached 13, 14, 15, 16 or 17 years of age during the pandemic (in 2020 or 2021) was similar to coverage before the pandemic (in 2019).
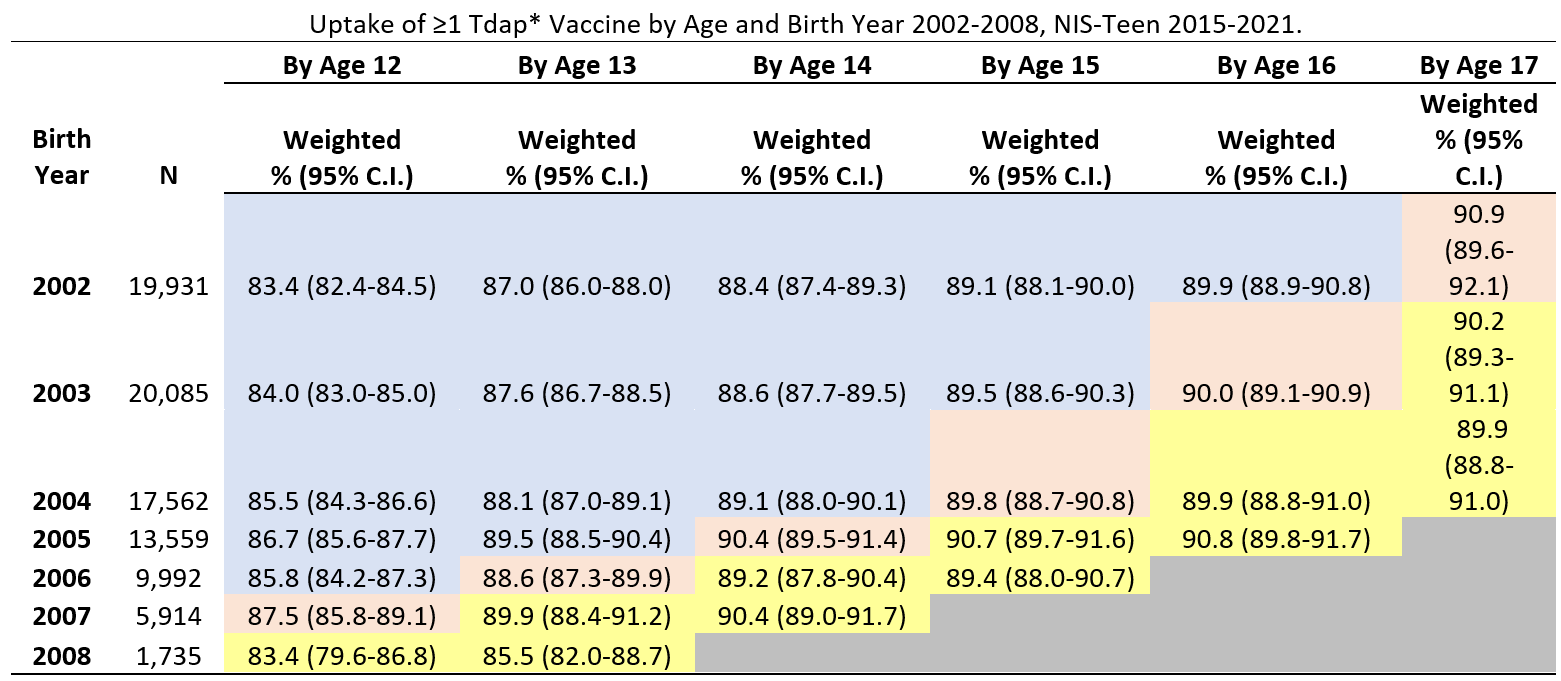
Abbreviations: Tdap = tetanus, diphtheria, and acellular pertussis vaccine; CI = confidence interval; NIS = National Immunization Survey.
* Includes percentages receiving Tdap vaccine at age ≥10 years.
† Table cells are shaded according to whether persons with the indicated birth year (rows) reached the age indicated (columns) in 2019 or earlier (pre-pandemic, shaded in blue or pink) or in 2020 or 2021 (during pandemic, shaded in yellow). Cells shaded in pink were used as the pre-pandemic referent cell for comparisons with pandemic cells, for each age indicated (column).
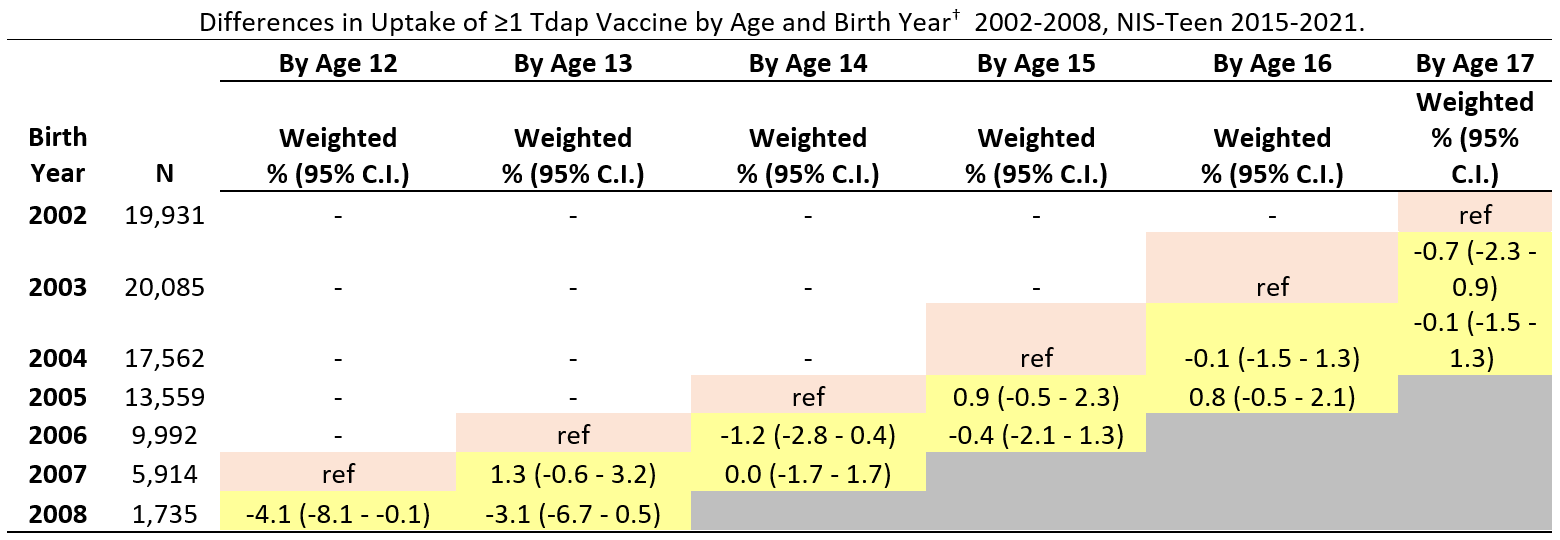
Abbreviations: Tdap = tetanus, diphtheria, and acellular pertussis vaccine; CI = confidence interval; NIS = National Immunization Survey.
* Includes percentages receiving Tdap vaccine at age ≥10 years.
† Reference birth years (denoted in pink cell shading) for vaccination coverage difference estimates by ages 14, 15, 16, and 17 years were the 2005, 2004, 2003, and 2002 birth years, respectively. Children in these reference birth years reached the given age in 2019 (e.g., for coverage by age 13 years, children born in 2006 reached 13 in 2019, which was before the pandemic). The reference pre-pandemic year (pink cell shading) is compared to pandemic years (2020 and 2021, yellow cell shading)
Coverage with ≥1 MenACWY dose among adolescents who reached 13 years of age during the pandemic (in 2021) was 5.1 percentage points lower (95% confidence interval, -9.8, -0.4) than among those who reached 13 before the pandemic (in 2019). MenACWY coverage among adolescents who reached 12, 14, 15, 16 or 17 years of age during the pandemic was similar to coverage before the pandemic.
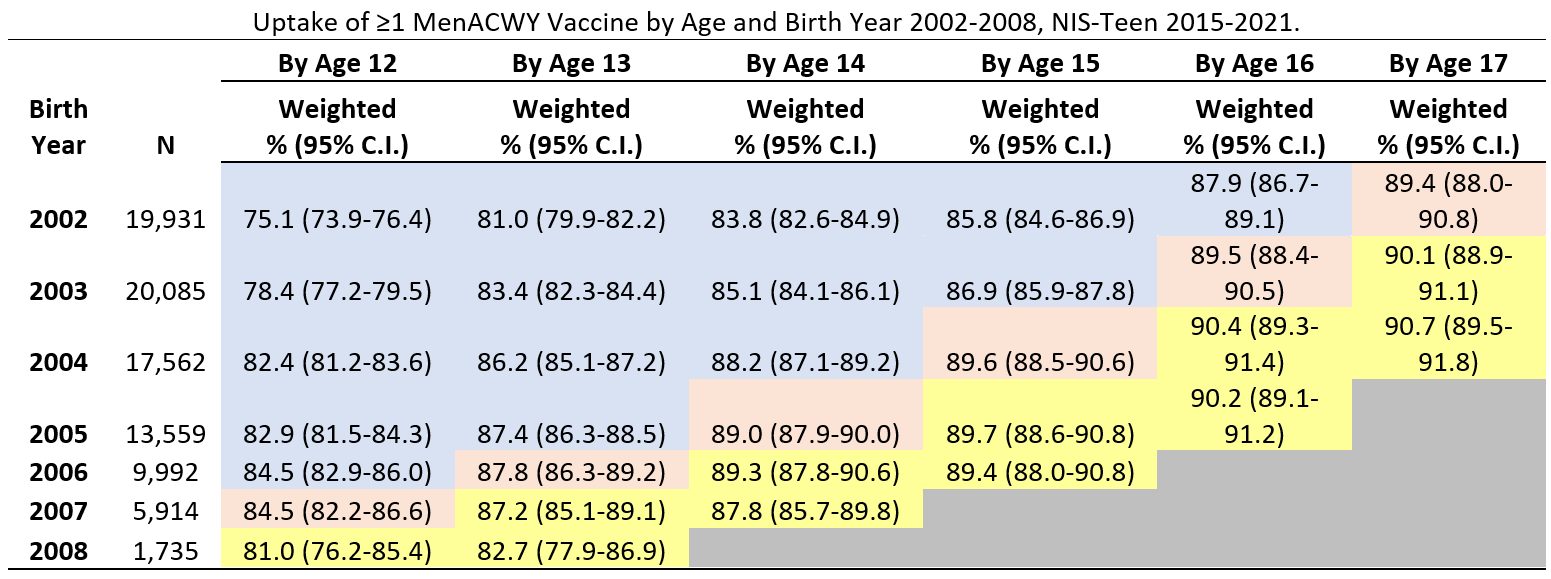
Abbreviations: MenACWY = quadrivalent meningococcal conjugate vaccine; CI = confidence interval; NIS = National Immunization Survey.
* Includes percentages receiving MenACWY and meningococcal-unknown type vaccine.
† Table cells are shaded according to whether persons with the indicated birth year (rows) reached the age indicated (columns) in 2019 or earlier (pre-pandemic, shaded in blue or pink) or in 2020 or 2021 (during pandemic, shaded in yellow). Cells shaded in pink were used as the pre-pandemic referent cell for comparisons with pandemic cells, for each age indicated (column).
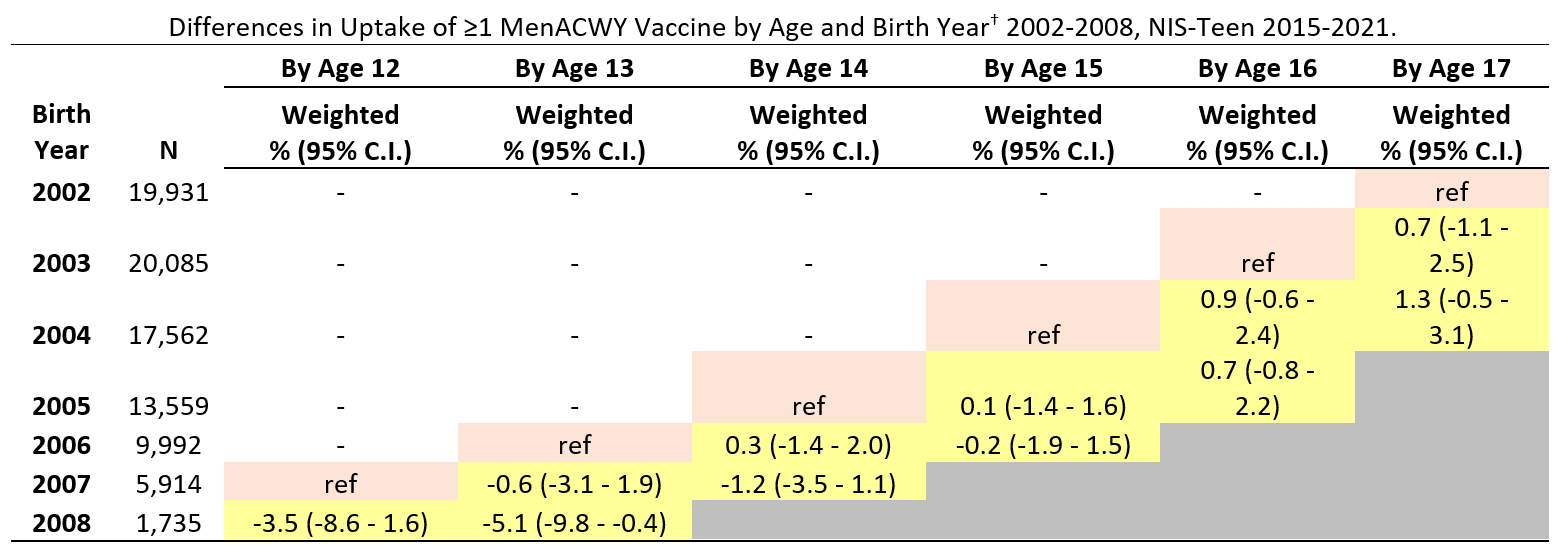
Abbreviations: MenACWY = quadrivalent meningococcal conjugate vaccine; CI = confidence interval; NIS = National Immunization Survey.
* Includes percentages receiving MenACWY and meningococcal-unknown type vaccine.
† Reference birth years (denoted in pink cell shading) for vaccination coverage difference estimates by ages 14, 15, 16, and 17 years were the 2005, 2004, 2003, and 2002 birth years, respectively. Children in these reference birth years reached the given age in 2019 (e.g., for coverage by age 13 years, children born in 2006 reached 13 in 2019, which was before the pandemic). The reference pre-pandemic year (pink cell shading) is compared to pandemic years (2020 and 2021, yellow cell shading)
The following graphs use estimates in the tables above to visually show trends by year of birth in coverage with ≥1 HPV vaccine, ≥1 MenACWY, and ≥1 Tdap by each age from 13 through 17 years. The vertical lines represent the pre-pandemic referent birth year; estimates to the right of this line are for adolescents who reached the indicated age during the pandemic (in 2020 or 2021).
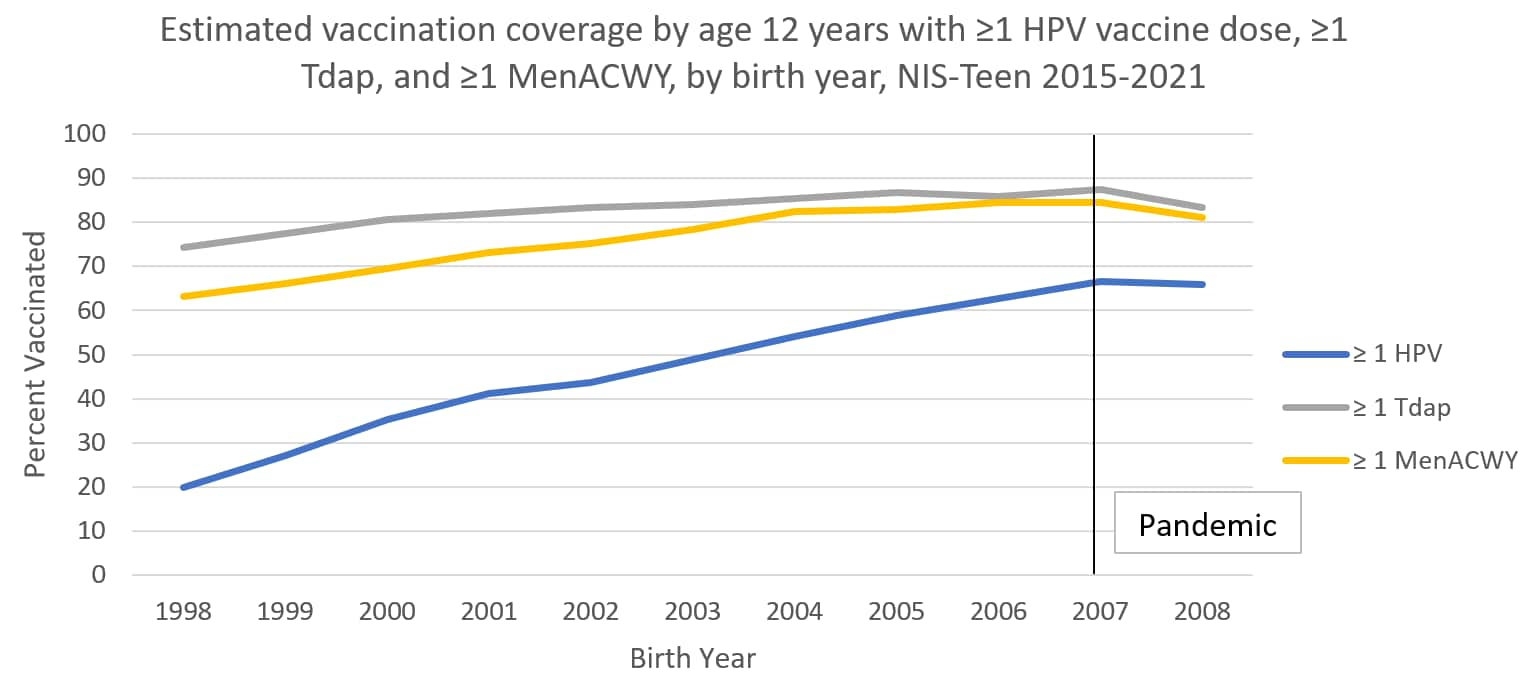
Abbreviations: HPV = human papillomavirus; Tdap = tetanus, diphtheria, and acellular pertussis vaccine; MenACWY = quadrivalent meningococcal conjugate vaccine; NIS = National Immunization Survey.
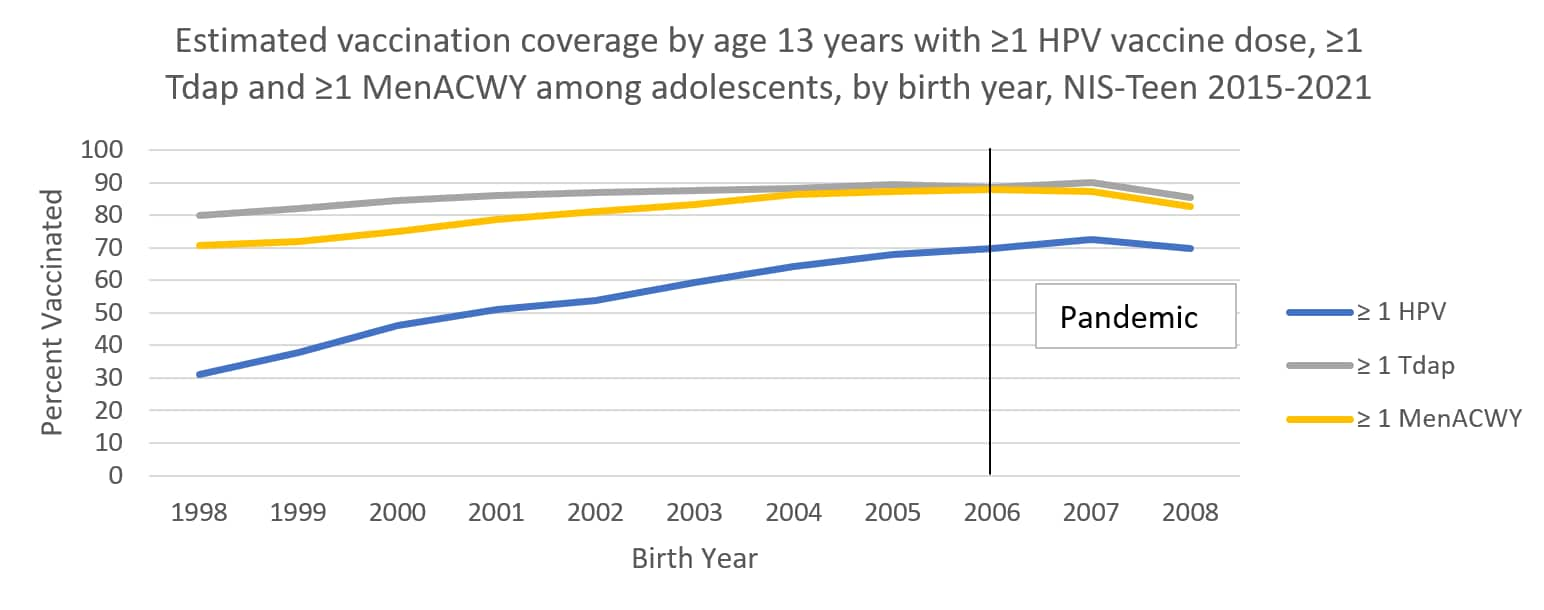
Abbreviations: HPV = human papillomavirus; Tdap = tetanus, diphtheria, and acellular pertussis vaccine; MenACWY = quadrivalent meningococcal conjugate vaccine; NIS = National Immunization Survey.
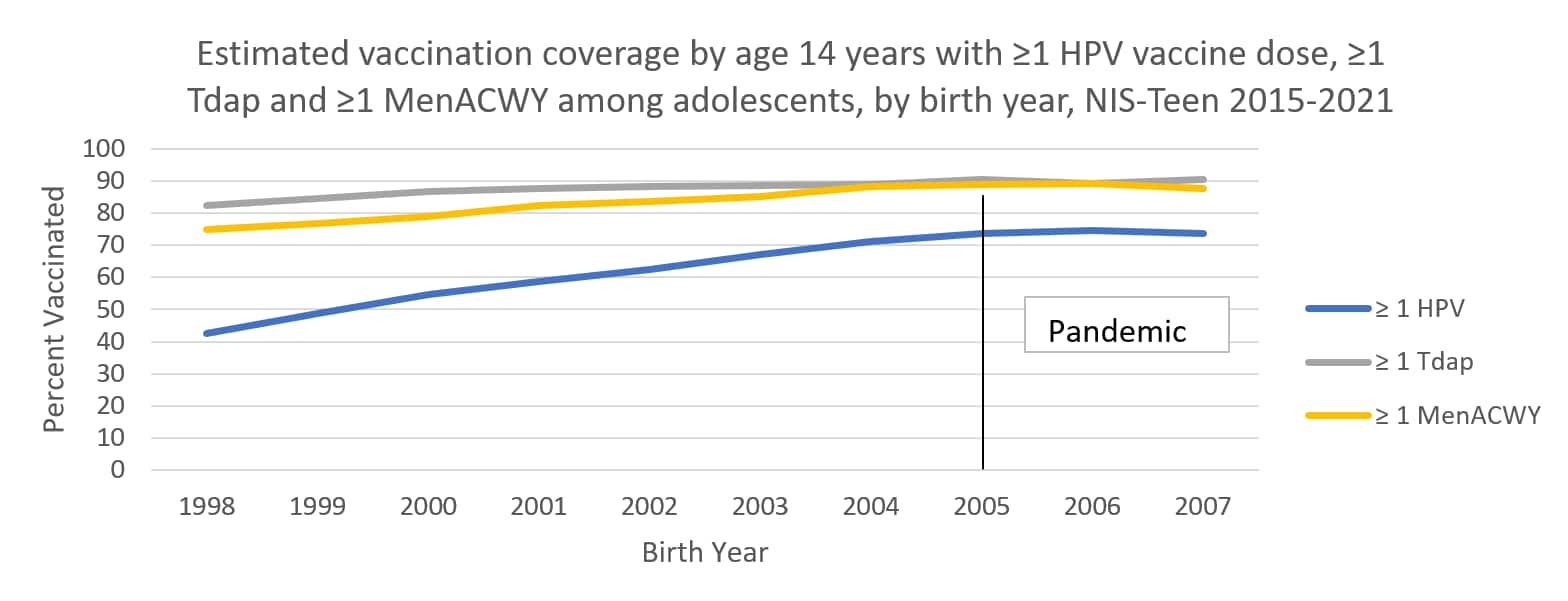
Abbreviations: HPV = human papillomavirus; Tdap = tetanus, diphtheria, and acellular pertussis vaccine; MenACWY = quadrivalent meningococcal conjugate vaccine; NIS = National Immunization Survey.
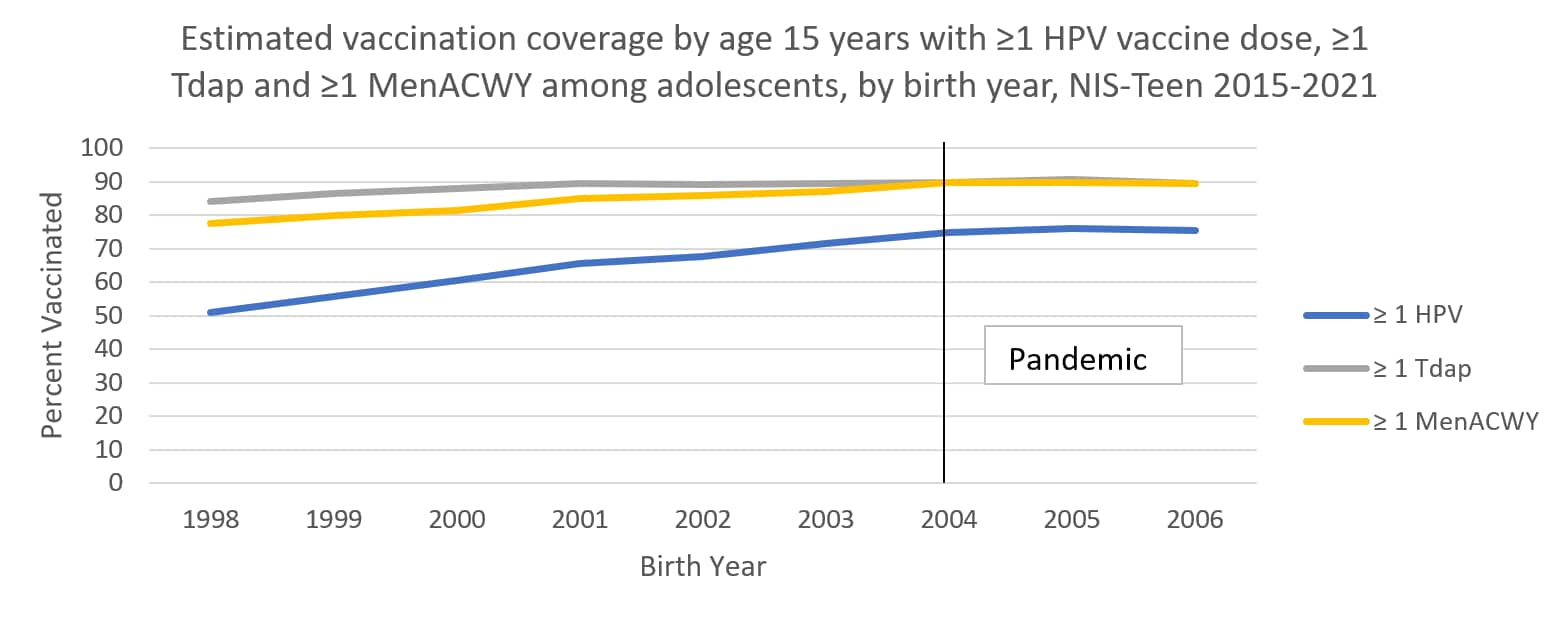
Abbreviations: HPV = human papillomavirus; Tdap = tetanus, diphtheria, and acellular pertussis vaccine; MenACWY = quadrivalent meningococcal conjugate vaccine; NIS = National Immunization Survey.
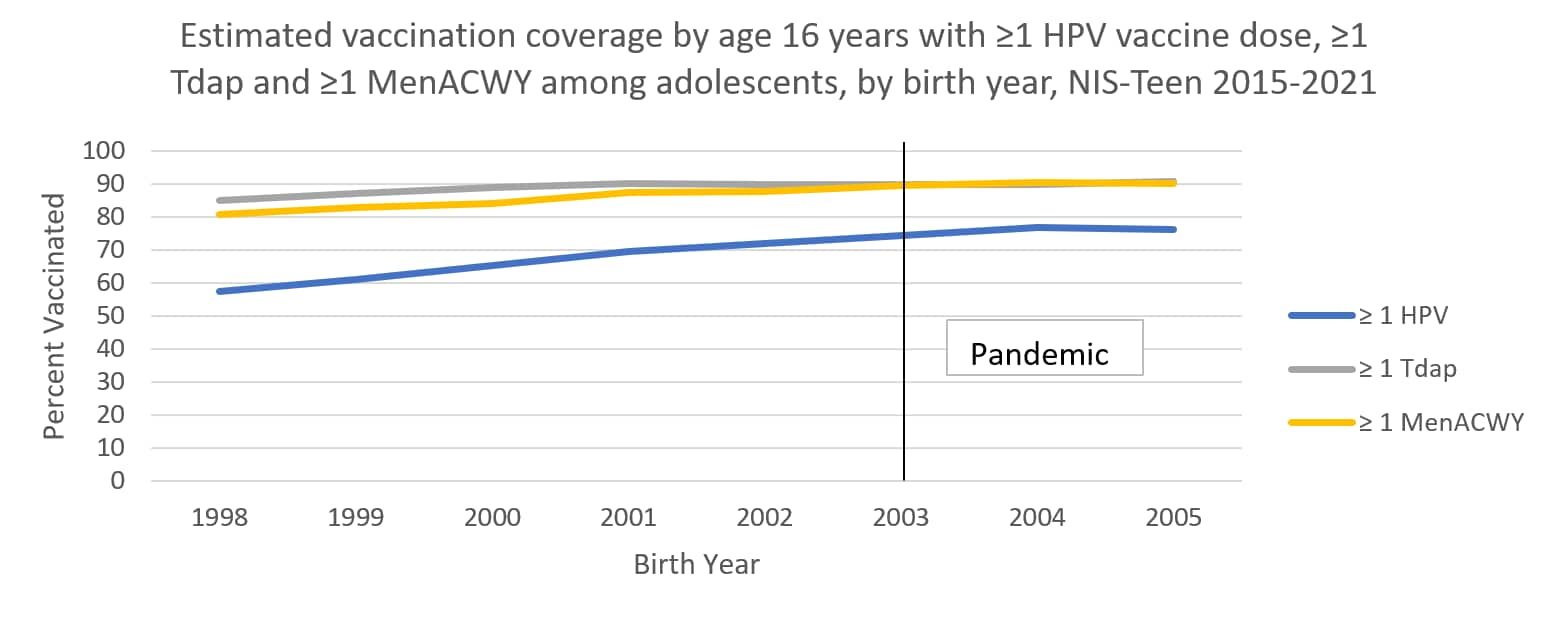
Abbreviations: HPV = human papillomavirus; Tdap = tetanus, diphtheria, and acellular pertussis vaccine; MenACWY = quadrivalent meningococcal conjugate vaccine; NIS= National Immunization Survey.
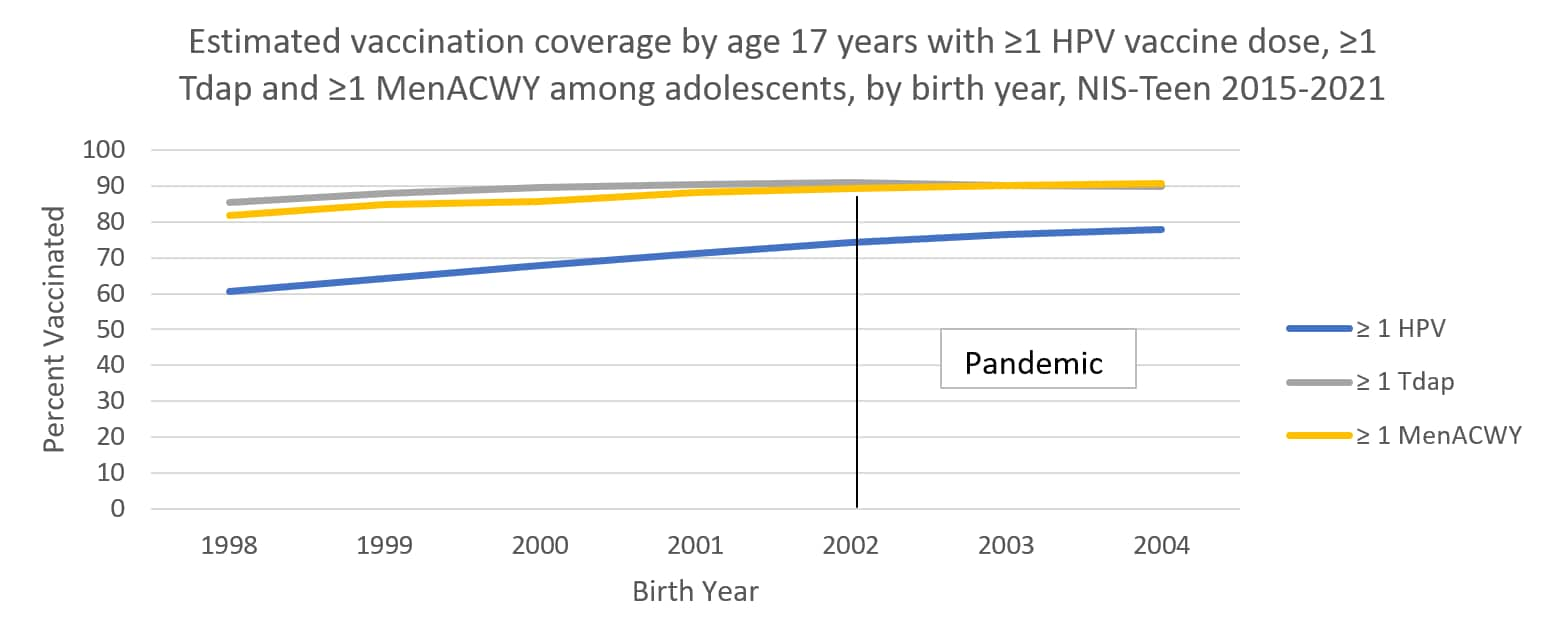
Abbreviations: HPV = human papillomavirus; Tdap = tetanus, diphtheria, and acellular pertussis vaccine; MenACWY = quadrivalent meningococcal conjugate vaccine; NIS = National Immunization Survey.
Discussion
Many adolescents may have missed routine medical care and recommended vaccinations during the COVID-19 pandemic in 2020A and 2021. Decreases in coverage with ≥1 MenACWY dose by age 13 years and Tdap by age 12 years for adolescents born in 2008 suggest that the COVID-19 pandemic has impacted vaccination coverage among adolescents due for routine vaccinations after 2019. Tdap coverage by age 13 years for adolescents born in 2008 was lower than for those born in 2006, but the difference was not statistically significant. Vaccination coverage at older age milestones (14-17 years) during the pandemic was not significantly different from coverage before the pandemic. As more adolescents who were aged 11–12 years during the COVID-19 pandemic in 2020 age into the NIS-Teen survey sample, the full impact of the COVID-19 pandemic can be better examined. To help adolescents catch up on missed vaccinations, healthcare providers can identify adolescents who have fallen behind on receiving recommended vaccinations and remind families to schedule an appointment. Additionally, during every clinical encounter, including those for COVID-19 vaccination, providers should review patients' vaccination histories and recommend needed vaccination. Resources to help promote and discuss vaccination with parents and patients can be found at www.cdc.gov/vaccines/hcp/patient-ed/index.html.
- An assessment of validity of the 2020 NIS-Teen estimates has been reported (www.cdc.gov/vaccines/imz-managers/nis/downloads/NIS-TEEN-PUF20-DUG.pdf). NIS-Teen vaccination coverage estimates tended to be slightly lower compared with true values derived after adjusting for noncoverage, nonresponse, and vaccination under ascertainment, reaching up to 6.3 percentage points too low for Tdap. This was primarily attributed to under ascertainment of vaccinations by the NIS provider record check. The validity of estimates did not change from 2019 to 2020.
- Wodi AP, Murthy N, Bernstein H, McNally V, Cineas S, Ault K. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger — United States, 2022. MMWR Morb Mortal Wkly Rep 2022;71:234–237. DOI:http://dx.doi.org/10.15585/mmwr.mm7107a2
- Pingali C, Yankey D, Elam-Evans LD, et al. National Vaccination Coverage Among Adolescents Aged 13–17 Years — National Immunization Survey-Teen— United States, 2021. MMWR Morb Mortal Wkly Rep 2022 DOI: www.cdc.gov/mmwr/index2022.html
