 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Experimental Surveillance Using Data on Sales of Over-the-Counter Medications --- Japan, November 2003--April 2004Yasushi Ohkusa, M. Shigematsu, K. Taniguchi, N. Okabe Corresponding author: Yasushi Ohkusa, Infectious Disease Surveillance Center, National Institute of Infectious Diseases, 123-1 Toyama, Shinjuku-Ku, Tokyo 162-8640, Japan. Telephone: 81-3-5285-1111; Fax: 81-3-5285-1129; E-mail: ohkusa@nih.go.jp. Disclosure of relationship: The contributors of this report have disclosed that they have no financial interest, relationship, affiliation, or other association with any organization that might represent a conflict of interest. In addition, this report does not contain any discussion of unlabeled use of commercial products or products for investigational use. AbstractObjectives: This report describes a study to explore the possibility of using data on sales of over-the- counter (OTC) medications as part of a routine syndromic surveillance system aimed at early detection of infections of public health concern. A retrospective evaluation was conducted of sales of OTC medications used to treat the common cold. This report discusses the correlation of these data to influenza activity in Japan during the 2003--04 influenza season and evaluates the potential of using such data to predict influenza epidemics. Methods: Data from approximately 1,100 pharmacies throughout Japan collected during November 2003--April 2004 were analyzed. OTC sales data were compared with influenza incidence data (one weekly and two daily data sets) to determine correlations and predictability. Adjusted R-square was used as an index of goodnessof-fit in the estimation. Data reflecting daily influenza activity were obtained from the National Surveillance of Daily Influenza Outpatients and the Mailing List--Based Influenza Epidemic Database. National sentinel surveillance data for influenza from approximately 5,000 sites nationwide also were analyzed. Results: Although a correlation was demonstrated between sales of OTC medications used to treat the common cold and concurrent influenza activity, analysis of sales data alone was not sufficient to determine influenza activity in advance even when sales promotion effects were excluded from the analysis. Conclusion: Because visiting a health-care provider costs more than purchasing OTC medications, the hypothesis was formed that an ill person will purchase OTC medications first and visit a physician only if the condition does not resolve or worsens. The results of this study do not provide any clear evidence to support this hypothesis. For this reason, OTC sales do not appear to be a good candidate for a national real-time detection system for influenza epidemics in Japan. IntroductionIn 2000, the first syndromic surveillance prototype in Japan was initiated by the Japanese Ministry of Health, Labour, and Welfare (MHLW) in the Kyushu area during the G-8 summit meeting to assist in the early detection of an act of biologic terrorism or an unusual cluster of tropical diseases imported by travelers from tropical areas (1). This limited-scale surveillance involved 17 medical institutes in two prefectures for <1 month. Data for the surveillance system were reported through facsimile transmissions for five syndromic categories (i.e., respiratory, gastrointestinal, neurological, cutaneous-mucous membrane-bleeding, and nonspecific). The second (and the first nationwide) syndromic surveillance system was implemented during May 20--July 14, 2002, in connection with the Japan-Korea 2002 World Cup soccer games. The Internet-based surveillance, which was conducted by MHLW and the Infectious Disease Surveillance Center of the National Institute of Infectious Diseases (NIID), grouped hospitalized patients by symptoms into the same five syndromic categories used in 2000. Both ad hoc syndromic surveillance systems operated during high-profile events and were conducted successfully, and their data were matched with those diseases with the same clinical features that were collected later by routine national surveillance. For example, this second ad hoc syndromic surveillance detected a cluster of viral meningitis and a regional outbreak of measles successfully, thereby illustrating the potential of these data in assisting with early detection of disease. However, further improvements are required to detect pandemic influenza or a possible biologic terrorist attack in time to minimize its consequences. The goal of the early detection syndromic surveillance system is to conduct routine (not ad hoc) surveillance that complements existing surveillance systems and to detect increases in the number of patients before they report to hospitals with severe conditions. Data concerning sales of over-the-counter (OTC) medications, emergency department (ED) visits, ambulance calls, and other factors were assessed as tentative candidates for early detection of disease outbreaks (2,3). Because no routine syndromic surveillance for respiratory syndrome had been conducted previously in Japan, the effectiveness of OTC surveillance in early detection was compared with multiple influenza surveillance systems that were already in place. This report presents interim findings from the OTC sales surveillance. MethodsData SourceCommercially available data collecting reported daily sales of OTC medications in all forms (e.g., tablets, powder, granules, and syrup) used to treat the common cold from 1,100 pharmacies throughout Japan were obtained. So-called combination or general common-cold medications were chosen for examination because use of such medications has long been accepted in Japanese society as the first and most common treatment for influenza-like illness (ILI). These medications usually consist of a combination of antipyretic analgesics (e.g., acetaminophen or ibuprofen), antitussives (e.g., dihydrocodeine phosphate or noscapine), expectorants (e.g., bromohexine hydrochloride, guaifenesin, or potassium guaiacolsulfonate), exogenous enzyme (e.g., lysozyme chloride), bronchodilator (e.g., dl-methylephedrine hydrochloride), antihistaminics (e.g., carbinoxamine maleate or mequitazine), vitamins (e.g., vitamin B1, B2, or vitamin C), and others (e.g., herbal medicines or caffeine). The category also includes combined herbal medicines that are licensed for common cold treatment. Data were collected by a private marketing company from randomly chosen pharmacies covering approximately 2.0% of the 50,000 pharmacies in Japan. The influenza season was defined as November--April. Sales data collected during November 2003--April 2004 were subjected to retrospective analysis to examine the suitability of OTC sales surveillance for early detection of unexpected rare events. OTC sales data were compared with reliable sentinel surveillance data for influenza collected during November 2003--April 2004 by the National Epidemiological Surveillance of Infectious Diseases (NESID) and with data on influenza activity collected daily by two other surveillance systems from clinics, hospitals, and health-care providers. In Japan, sentinel reporting of clinical cases of ILI is mandatory, with or without laboratory tests or confirmation. Data (e.g., the number of influenza outpatients, by age and age group) are collected weekly from 5,000 sentinel surveillance sites (including 3,000 pediatricians and 2,000 internal medicine clinics or departments) nationwide covering one tenth of all clinics and hospitals in Japan for all influenza-related visits. Two daily influenza activity information sources are 1) reported numbers of cases of ILI reported by the National Surveillance of Daily Influenza Outpatients (Daily Case Reporting [DCR]), which collects data from 10% of selected sentinel medical institutions and 2) voluntary reporting by clinicians to the Mailing List--Based Influenza Epidemic Database (MLflu). DCR is operated by NIID and began operating in January 2004 for the 2003--04 influenza season; it collects data regarding the number of outpatients who received a diagnosis of ILI either clinically or by diagnostic test from 500 sentinel sites in clinics and hospitals. Date of onset is not included in the reported data, which makes this surveillance vulnerable to the-day- of-the-week effect (i.e., few patient visits reported during the weekend and more on the following Monday). MLflu is operated by volunteer pediatricians and began operating in December 2003 for the 2003--04 influenza season; it collects data from approximately 350 pediatricians regarding outpatients who have received a diagnosis of influenza by rapid test. Cases reported through MLflu are more likely to reflect actual influenza activity. Date of onset is reported, so the surveillance system is free from the-dayof-the-week effect. However, because reporting is voluntary, the number and representativeness of participants varies during the influenza season. AnalysisA model was created to estimate influenza activity from the OTC sales information during a 6-month period, as follows:
OTC sales data were then adjusted for the-day-of-the-week effect and compared with three other different influenza activity surveillance systems (sentinel surveillance, DCR, and MLflu) to examine the number of lead-days by OTC sales. The adjusting procedure consisted of two steps, as follows: The data set was adjusted by replacing data for weekends, holidays, and the day before and after weekends or holidays with data for the nearest preceding nonholiday weekday. Then the replaced data were smoothed to the past by taking a moving average from the current period to 1 week previous, giving a relatively heavier weight to the nearer days, and gradually reducing the weight for the far past. Although this adjusting procedure did not require future data, the adjustment result might be affected (pulled) from the data used for the replacement and smoothing procedure. Comparative analysis of OTC sales with one weekly and two daily data sets recording influenza incidence was performed to determine correlations and predictability. Adjusted Rsquare was used as an index of goodness-of-fit in the estimation. ResultsBecause national surveillance data do not capture the number of persons who consult a health-care provider for general respiratory symptoms, data regarding consultations for influenza symptoms were used as a substitute to assess lead time of OTC information. Influenza surveillance in Japan was designed to report all potential influenza patients from at least one system for robust detection of influenza activity other than hospitalization (Figure 1). The case definition of influenza used for both outpatient sentinel surveillance and DCR was based on clinical symptoms, which resulted in reporting of patients with ILI. For this analysis, the hypothesis used was that the majority of persons who were infected by influenza virus and who experienced mild symptoms would choose to self-treat with OTC medications and that those persons whose condition subsequently became more serious would then consult a physician later. Data of sales of OTC medications used to treat the common cold, readily provided as commercial databases, were assumed to reflect the population of preclinical visits by persons with ILI. Data on outpatient visits were represented by sentinel surveillance, DCR, and MLflu. An increase in OTC sales of medications used to treat the common cold was assumed to indicate an initial increase of ILI, and the lead time of the sales to the influenza activity was expected to be observed. OTC sales per pharmacy were tracked, and the time trend of sales per pharmacy, which was adjusted for the-day-ofthe-week effect and then smoothed, was given as a line (Figure 2). Multiple peaks of different size were observed during the 5-month surveillance period, with the consistent underlining trend being that sales were higher in winter and decreased toward spring. Peaks observed were in early and mid-December, early February, and late March. The third peak observed occurred during late January--early February and corresponded with the peak of ILI sentinel reporting generally recorded during influenza seasons; a subsequent period of decline toward spring was also matched. However, the pattern of the early influenza season was fairly discrete between the two data sets (Figure 3). Adjusted OTC sales data also were compared with adjusted influenza data from DCR to identify a similar pattern during the height of the influenza season (Figure 4). DCR for clinically confirmed ILI is case-based and includes the patient's age and age group, date of visit, performance of rapid test, and result of a rapid diagnostic test as a single thread of information. Because data are reported by clinics and hospitals, numbers were low on Saturdays and Sundays and high on Mondays; consequently, numbers were adjusted for the-day-of-the-week effect. As with sentinel surveillance, DCR also indicated a different pattern early in the influenza season, and the peak coincided with the third peak of OTC sales. Characteristically, no rise in DCR was observed to match the last peak of OTC sales during late March. MLflu data were reported voluntarily by physicians interested in influenza preparedness. Information collected through the case-based reporting system included the patient's age, date of illness onset, date of visit, type of rapid diagnostic test used, type of influenza virus (A or B) diagnosed, and name of antivirals or other common cold medications prescribed. The date of onset was available for MLflu, which made it free from the-day-of-the-week effect. Additionally, this system was able to provide the number of laboratory-confirmed cases of influenza (i.e., those diagnosed by rapid diagnosis tests). A limitation of this system was that the number of participants varied during the season (low at the beginning and the end of the season). Interest of the clinicians participating in MLflu was high when ILI was rapidly increasing but decreased after the peak period ended (Figure 5). The effect of this variance in the reporting rate should be considered when interpreting the results. As with the other two influenza surveillance systems, MLflu indicated a different pattern from the OTC medicine surveillance at the beginning of the influenza season (Figure 5). However, for the third peak, the rise in sales of OTC medications did not coincide with the peak of MLflu reporting. Instead, the peak observed by MLflu preceded sales by 1--2 weeks (Figure 5). No matched peak was observed for the one during March. OTC sales data were compared with other influenza activity surveillance data to determine lead time (i.e., the number of days that OTC sales elevation preceded an increase in the number of influenza patients) (Figure 6). Fitness among DCR declined as lead time became longer. The highest adjusted R-square was obtained when OTC data led by 1 day. Conversely, fitness among sentinel surveillance or MLflu rose when lead time was longer. In the case of sentinel surveillance or MLflu, OTC sales appeared to lag behind influenza activity. A peak in OTC sales observed at the end of 2003 was suspected to reflect influenza activity. DiscussionSyndromic surveillance in Japan has been conducted on an ad hoc basis during high-profile events (1). A short-term, labor-intensive analysis system was used that was expensive and resource-intensive to run on a daily basis. To date, several routine influenza surveillance systems have been implemented in Japan. However, each system by itself is unable to provide sufficient information to prepare for the potential emergence of pandemic influenza or related diseases. None of three currently existing influenza surveillance systems might be able to detect the early stage of a pandemic because all systems detect patients only at the point of consultation. In addition, each surveillance system has certain limitations. For example, the national sentinel surveillance provides reliable mandatory reporting but captures only the number of patients who visit sentinel clinics and hospitals without collecting sufficient qualitative information. These data are reported weekly, with a 1-week delay during which data are compiled. DCR captures additional qualitative information but reports include only the date of visit. MLflu reports the number of patients who receive a diagnosis for influenza with rapid testing. In each surveillance system, timeliness, accuracy, and representativeness have been traded off for other advantages. The rationale for using readily available data of OTC sales for monitoring is to establish routine early detection surveillance for pandemics and other unexpected events to complement those surveillance systems. The lead time for OTC sales was compared with influenza surveillance to evaluate the timeliness of sales data for detecting seasonal influenza epidemics. An estimated 72% of Americans with cough, cold, influenza, or sore throat often purchase OTC medications early in the course of their illnesses (4). Increases in OTC sales were expected to precede an increase in patient visits to hospitals, assuming that consumer behavior in Japan is similar to that in other developed countries (i.e., persons purchase OTC medications when they first feel ill and then visit clinics or EDs if their illness becomes more serious). Although OTC sales correlated well with contemporary influenza activity (2,3), a clear lead time was lacking, and analysis of OTC sales data indicated no evidence of advance detection of influenza activity. Additionally, difficulties were encountered in interpreting sales increases in late December from influenza surveillance alone. The increase appeared to reflect preparation for a long holiday season accelerated by year-end discount promotions but not an actual increase in influenza activity. However, further analysis excluding this sales promotion effect was also not able to determine any influenza activity in advance. These results indicate that sales data on OTC medications used to treat common colds have a low potential for predicting increased influenza activity in Japan. Multiple factors might account for this outcome. Because the analysis was performed only on a national level, the study did not take into account regional variations in influenza activity and variations in when the influenza season began. Variation of lead time of OTC sales to the actual disease incidence by locality has been suggested previously (5); therefore, to assess the real situation, smaller geographic areas must be analyzed. The next step to confirm correlations will be to break down the analysis at the prefecture level for 47 prefectures, with and without the effects of sales promotions. However, commuters cross prefecture borders frequently every day, and spatial correspondences or noncorrespondences of OTC sales and physician visits might remain biased in certain instances as a result of inexact geographic data. The choice of OTC medications selected for this study might have contributed to the outcome. The study was limited to medications used to treat the common cold, which were already grouped in the commercialized sales reporting database. However, in certain cases of early stages of influenza, persons might purchase more symptom-oriented medications (e.g., antipyretic analgesic, antitussive, and antihistaminic medications). To include the entire sales rise attributable to ILI in the analysis, medications in those categories should be examined to formulate a suitable product group to use as precursor for detecting increased ILI as soon as data become available (5). As the copayment proportion of payment for medical care by consumers continues to rise, a gradual move toward self-medication is under way in Japan. Consequently, the potential value of using OTC medication sales data as an indicator of disease outbreaks should continue to rise. However, Japanese consumers are still relatively reluctant to take an active role in decision making regarding their own health care. In addition, the majority of Japanese have easy access to medical care, and the national health insurance system provides a high degree of coverage. As a result, persons who are ill are more likely to visit a clinic at an early stage of illness. The introduction of antiviral agents (e.g., oseltamivir) that require a physician's prescription also has promoted medical assistance--seeking behavior during the influenza season. All of these factors combined might have influenced the study results. ConclusionThe results presented in this report are tentative. Thorough data cleaning and additional analysis are required before a final decision is made concerning the use of OTC medication sales data as part of a national real-time syndromic surveillance system. Further studies are planned, including a geographic breakdown analysis, analysis with exclusion and inclusion of sales promotion effects (other than the year-end discount promotion), choice of methods for statistical analysis, and analysis taking into account bargain sales and associated promotion types and trial surveillance concerning respiratory symptoms in a limited area. Acknowledgments Naruo Saito and physicians participating in MLflu surveillance provided data for the 2003--04 influenza season. Shizuka Matsuoka provided technical support for a poster presentation of this research. The research was funded by a grant from the Ministry of Health, Labour, and Welfare, Japan. References
Figure 1 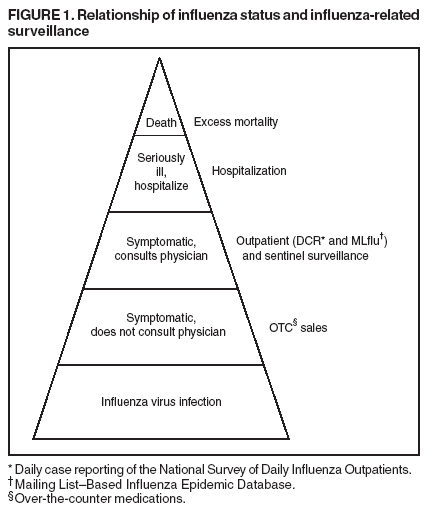 Return to top. Figure 2 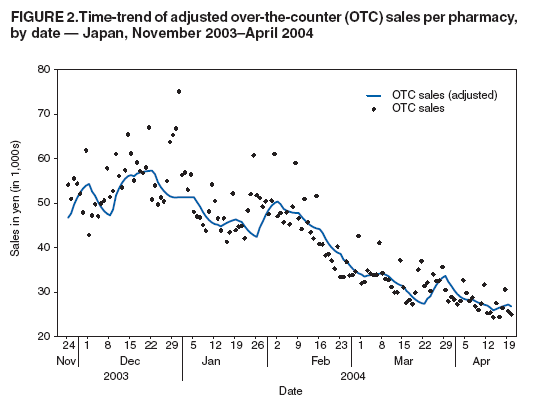 Return to top. Figure 3 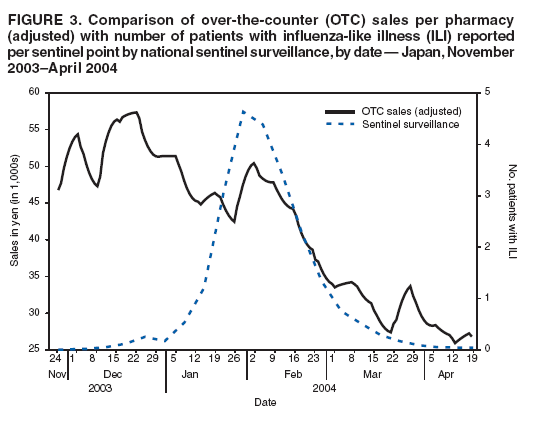 Return to top. Figure 4 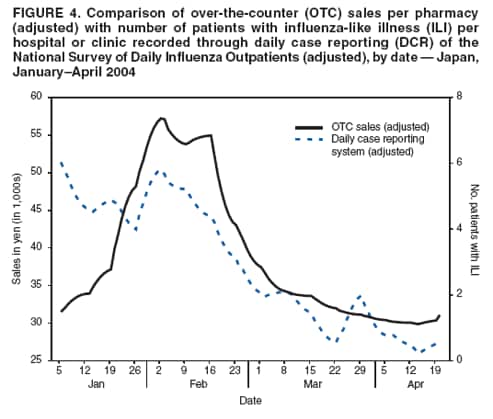 Return to top. Figure 5 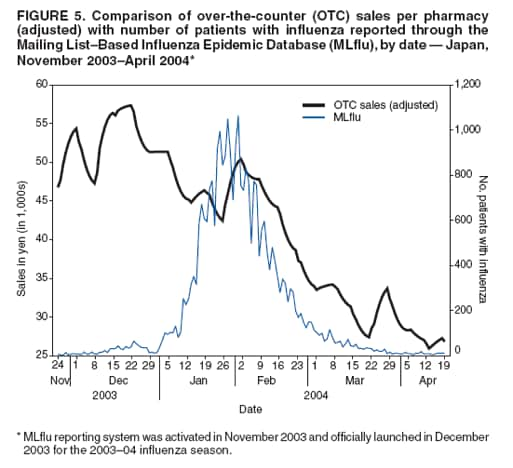 Return to top. Figure 6 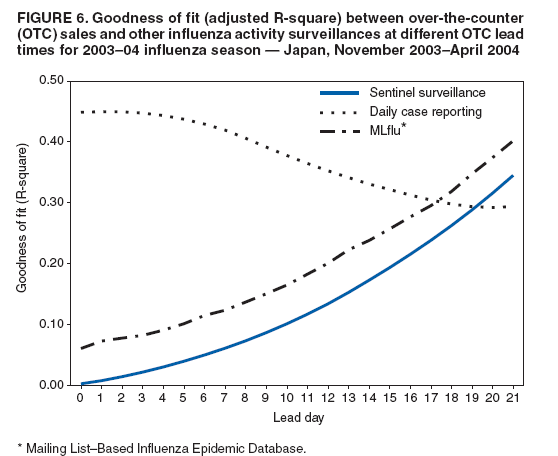 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 8/5/2005 |
|||||||||
|