 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Two Cases of Hantavirus Pulmonary Syndrome --- Randolph County, West Virginia, July 2004Hantavirus pulmonary syndrome (HPS) is a rare cardiopulmonary disease caused by viruses of the genus Hantavirus, for which rodents are the natural reservoir (1,2). Transmission to humans occurs by direct contact with rodents or their excreta or by inhalation of aerosolized infectious material (e.g., dust created by disturbing rodent nests). In July 2004, HPS cases (including one fatal case) were reported in two persons believed to have been exposed at sites approximately 12 miles apart in Randolph County, West Virginia (2000 population: 28,254) (3). This report describes the two cases and summarizes their epidemiologic and environmental investigations. Clinicians and the public need to be educated about the risk for HPS and methods to reduce that risk. Case InvestigationsPatient A. In early July, a wildlife sciences graduate student, a man aged 32 years, visited an emergency department (ED) in Blacksburg, Virginia, with complaints of fever, cough, and sore chest since the previous evening. The ED clinician noted possible rodent exposure in the medical history of the patient. Examination revealed a temperature of 102.7°F (39.3°C) and an oxygen saturation of 96% (normal). A complete blood count (CBC) revealed a left shift with no bands (granulocytes: 87%) and lymphopenia (lymphocytes: 400/mm3). Radiographic examination indicated faint right-sided pneumonia. In the ED, the graduate student began vomiting and was admitted for intravenous hydration and parenteral antibiotics. He became progressively hypoxic, requiring supplemental oxygen, bilevel positive airway pressure, and eventually intubation with mechanical ventilation. Repeated radiographs revealed bilateral pulmonary edema. The next day, the patient was hypotensive, requiring intravenous pressor support. He received activated protein C to prevent disseminated intravascular coagulation (DIC). A repeat CBC revealed bands (granulocytes: 20%) and a decreased platelet count (115,000/mm3); urinalysis indicated mild hematuria and proteinuria. Despite aggressive supportive care, the patient's status continued to deteriorate, and he died on the third day of his hospitalization. Differential diagnosis included tularemia, pneumococcal sepsis, and HPS. Serum specimens submitted to ARUP Laboratories (Salt Lake City, Utah) were positive for both IgG and IgM antibodies to hantaviruses; these test results were confirmed by CDC. A spleen biopsy was also positive by immunohistochemistry for hantavirus antigens. A serum sample was positive for hantavirus RNA by real-time reverse transcriptase-polymerase chain reaction (RT-PCR). Sequencing of the amplified nucleic acid identified the virus as Monongahela hantavirus (4). According to interviews with his coworkers, the patient had spent the previous month trapping small mammals for study and handling mice (Peromyscus spp.) daily. Two students and a recent graduate who had worked with the patient reported that none of them had consistently worn gloves while handling rodents or washed their hands after handling rodents or their excreta, even before eating. The students also reported frequent rodent bites on their bare hands. Patient B. In early July, a Randolph County resident, a man aged 41 years, spent a weekend at a log cabin with his family. Two days later, he had fatigue, a dull headache, and a mild fever. The following day, he had a temperature of 102.9°F (39.4°C). The next morning, he visited his primary-care physician with hematuria but no fever and was released on empiric antibiotic therapy for a possible urinary tract infection. The patient returned 2 days later with a severe headache of approximately 12 hours' duration; he was referred immediately to the local ED. On arrival, the patient was hypoxic with a room air oxygen saturation of 90%; chest radiographs revealed right-sided pneumonia and congestive heart failure. The patient was airlifted to a referral hospital, with hypotension and bradycardia. His white blood cell count was normal, and cardiac enzymes were negative. The patient was placed in the intensive care unit and administered intravenous pressors and broad-spectrum antibiotics. Differential diagnosis included viral myocarditis, atypical pneumonia, and opportunistic infection, and was later broadened to include HPS and other infectious and autoimmune etiologies. The patient was intubated the next day and started on high-frequency oscillator ventilatory support. The patient's condition deteriorated, with onset of thrombocytopenia, DIC, hypoalbuminemia, and renal insufficiency requiring hemodialysis. After 5 days of hospitalization, his condition began to improve. Serum samples were reported positive for IgG and IgM antibodies to hantaviruses by ARUP Laboratories; these results were confirmed by CDC. In addition, a serum sample taken during his hospitalization was positive for hantavirus RNA by RT-PCR. Sequencing of the amplified nucleic acid also identified the virus as Monongahela hantavirus. The patient recovered slowly during the next month. According to family members, when the patient and his family arrived at the cabin in early July, they aired the interior after finding it reeking of rodent urine and discovered two live mice in a trash can in the kitchen. The patient killed the mice and later disposed of the remains and cleaned the trash can without wearing gloves. The family slept in the cabin that weekend and trapped six additional mice during their stay. Environmental InvestigationOn August 3, investigators from CDC and the West Virginia Department of Health and Human Resources discovered additional live mice in the trash can in the cabin of patient B. Openings in the walls and eaves were identified that permitted easy entry by rodents. In all, rodents were trapped by the investigating team during August 3--6 from three rural sites in Randolph County: 1) the dormitory in which patient A lived and its surroundings, 2) a forest trapping site where patient A worked the week before his illness, and 3) the family cabin and surroundings of patient B. Fourteen white-footed mice (P. leucopus) and one deer mouse (P. maniculatus) were captured from 239 traps during a 3-day period. Tissue and blood specimens were collected and processed for serology. RT-PCR was conducted on specimens of rodents with positive serology results. Hantavirus antibodies were detected in one white-footed mouse, which was also positive for virus RNA by RT-PCR. Sequence of the amplified RNA indicated that the mouse was infected with Monongahela hantavirus identical to virus identified in rodents collected from the location where patient B was presumed to have been infected. The amplified nucleic acid sequence was similar, but not identical, to that amplified from patient A. Reported by: Randolph County Dept of Health; J Rooney, DVM, West Virginia Dept of Health and Human Resources. K McCombs, MPH, New River Health District, Virginia Dept of Health. Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; B Pavlin, MD, J Sinclair, DVM, EIS officers, CDC. Editorial Note:Since HPS was first identified in the southwestern United States in 1993, a total of 379 laboratory-confirmed cases of HPS have been reported in the United States, including 32 retrospectively identified cases that occurred before 1993. Cases have been reported in 31 states, the majority of cases in the Southwest. Three cases of HPS have been identified as acquired in West Virginia. Subclinical infections are rare, according to antibody prevalence studies performed after the 1993 outbreak (5--7). In the first case described in this report, exposure was probably occupational. Patient A regularly handled multiple mice, often suffered bites, and reportedly did not routinely wash his hands after handling rodents. In the second case, the exposure was peridomestic, likely associated with contact with live mice and their excreta while removing them from his cabin. Despite the temporal and geographic proximity of the two cases, no common exposure source, other than the rodent contact described, appears to exist. These cases underscore the need to educate the public and clinicians about the risk for HPS in areas outside the Southwest. In addition, persons who have occupational exposure to rodents and their excreta should be trained in proper animal handling and use of personal protective equipment. Simple, effective methods are available to reduce exposure to hantaviruses (Box). Adherence to these precautions can reduce the incidence of HPS. Acknowledgments The report is based on data provided by P Keyser, PhD, MeadWestvaco Corporation, Elkins; M Fisher, MD, Ruby Memorial Hospital, Morgantown; J Crum, PhD, West Virginia Div of Natural Resources. M Kelly, PhD, Dept of Fisheries and Wildlife Science, Virginia Polytechnic Institute and State Univ, Blacksburg, Virginia. References
Box 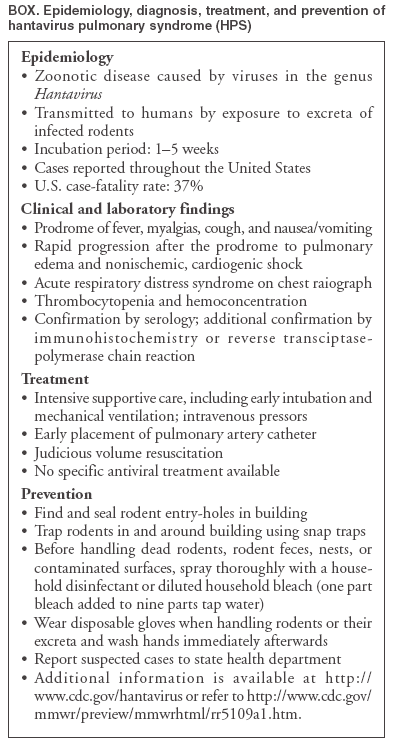 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 11/23/2004 |
|||||||||
This page last reviewed 11/23/2004
|