 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Imported Lassa Fever --- New Jersey, 2004Lassa fever is an acute viral illness caused by Lassa virus, which is hosted by rodents in the Mastomys natalensis species complex and rarely imported to countries outside of those areas in Africa where the disease is endemic (1). Lassa fever is characterized by fever, muscle aches, sore throat, nausea, vomiting, and chest and abdominal pain. Approximately 15%--20% of patients hospitalized for Lassa fever die from the illness; however, approximately 80% of human infections with Lassa virus are mild or asymptomatic, and 1% of infections overall result in death (1). On August 28, 2004, a man aged 38 years residing in New Jersey died from Lassa fever after returning from travel to West Africa. This report summarizes the clinical and epidemiologic investigations conducted by federal, state, and local public health agencies. The findings illustrate the need for clinicians and public health officials to remain alert to emerging infectious diseases and to institute appropriate measures to promptly identify and limit spread of unusual pathogens. Case ReportThe patient, a businessman who was born in Liberia, had resided in the United States for 5 years. During the 4-month period preceding hospitalization, he had been in West Africa, commuting frequently between Liberia and Sierra Leone, where he owned farms. One day in August, the patient began to experience fever, chills, severe sore throat, diarrhea, and back pain. Two days later, he left Freetown, Sierra Leone, and traveled by airplane through London, England, arriving in Newark, New Jersey. He then traveled from Newark to his home by train. Within hours of his arrival in the United States, the patient sought treatment and was hospitalized in Trenton, New Jersey, for persistent fever, chills, sore throat, diarrhea, and back pain. On admission, the patient was alert and had a temperature of 103.6ºF (39.8ºC). Differential diagnoses at this time included malaria and typhoid fever. On the third and fourth days of hospitalization, despite treatment with antimalarial and antibiotic therapy, the patient's condition deteriorated, and adult respiratory distress syndrome was diagnosed. He was subsequently intubated and mechanically ventilated. Yellow fever and Lassa fever were considered as possible diagnoses. The New Jersey Department of Health and Senior Services (NJDHSS) was notified, CDC was consulted, and arrangements to administer intravenous ribavirin under an investigational new drug protocol were initiated. However, 6 hours later, the patient died before the drug could be administered. Clinical and postmortem specimens were sent to CDC for specific diagnostic testing. Lassa fever was confirmed by using serum antigen detection, immunohistochemical staining of postmortem liver-biopsy specimens, virus isolation in cell culture (Figure 1), and sequencing of Lassa virus by reverse transcriptase-polymerase chain reaction. InvestigationAn investigation was conducted to identify persons who might have had direct contact with the patient or his body fluids while he was ill. Contacts were categorized into low- and high-risk categories on the basis of multiple criteria (Box). A total of 188 persons had contact with the patient during the period when he was likely infectious; of these, five persons were classified as at high risk and 183 as at low risk. The five at high risk were the patient's wife, three of their children, and the patient's brother, who was a hospital visitor; each reportedly had unprotected exposure to the patient's body fluids during his illness. Contacts at low risk included nine other family members, 139 health-care workers employed at the Trenton hospital (including 42 laboratory workers, 32 nurses, and 11 physicians), and 16 laboratory workers employed at commercial laboratories in Virginia and California. In addition, 19 contacts at low risk were exposed as passengers on the flight from London to Newark. The NJDHSS notified CDC's Division of Global Migration and Quarantine (DGMQ) of possible travel-related exposures. Because the patient reported illness onset 3 days before air travel, DGMQ searched for those airline passengers who had been seated within 6 feet of the patient. Passengers were traced by using information from travel reservation records and customs declaration forms. Nineteen passengers seated near the patient were identified (Figure 2). Within 5 days of notification, 13 of the 19 passengers had been interviewed; within 8 days, three more had been contacted. The remaining three could not be contacted. Seventeen of 19 passengers were citizens of the United Kingdom, and authorities in that country were notified; two of the passengers were U.S. citizens. Interviewed passengers did not report contact with the patient's body fluids and were considered to have low-risk exposure. All passengers contacted were healthy; none reported fever as of September 14, which marked the end of the 21-day incubation period for Lassa fever for this group. All contacts at high risk (i.e., five family members) were monitored for temperature of >101ºF (>38.3ºC) twice daily for 21 days after their last potential exposure to the patient on August 28. A public health nurse visited the family contacts each morning and recorded their temperatures. In the afternoon, the contacts recorded their own temperatures and reported the results. The majority of contacts at low risk (i.e., nine other family members and 139 health-care workers) were instructed to record their own temperatures at least twice daily and report the results. Other contacts at low risk (i.e., the 16 laboratory workers and 19 air passengers) were asked to self monitor for temperature of >101ºF (>38.3ºC) and other symptoms compatible with Lassa fever. No restriction was placed on work or movement for asymptomatic adults at either high or low risk. However, to facilitate monitoring, the patient's children were restricted from participating in school activities. None of the contacts at high risk reported any illness compatible with Lassa fever as of September 18, which ended their 21-day incubation period. Reported by: P Aufiero, MD, N Karabulut, MD, D Rumowitz, S Shah, DO, Capital Health System, Trenton; J Nsubuga, MPH, B Piepszak, RD Salter, MA, City of Trenton Div of Health; E Bresnitz, MD, CR Lacy, MD, C Robertson, MD, C Tan, MD, New Jersey Dept of Health and Senior Svcs. Div of Global Migration and Quarantine, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; ET Tan, MBBS, EIS Officer, CDC. Editorial Note:This report describes the first case of imported Lassa fever diagnosed in the United States since 1989 (2). In West Africa, Lassa fever is endemic, causing 100,000--300,000 human infections and approximately 5,000 deaths each year (1). Other than in regions where it is endemic, Lassa fever is encountered rarely. Cases identified in areas where Lassa fever is not endemic usually are imported, often by persons returning from West Africa (2). To date, approximately 20 cases of imported Lassa fever have been reported worldwide. The risk for human-to-human transmission of Lassa fever is low (3,4); however, health-care--associated transmission has occurred in areas where Lassa fever is endemic, and one instance of asymptomatic seroconversion was reported in a European physician (4). Meticulous adherence to appropriate infection-control practices to prevent unprotected exposure to blood or other body fluids is essential to the safe management of patients with possible Lassa fever and to the protection of health-care workers (2). Family members and others visiting a hospitalized patient must be instructed to adhere to infection-control precautions and avoid exposure to potentially infectious blood or body fluids. In the absence of proven effectiveness, oral ribavirin prophylaxis was not recommended for persons who might have been exposed to the patient described in this report. Instead, a standard treatment regimen of intravenous ribavirin was recommended for any contacts with clinical evidence of infection during the incubation period. However, none of the contacts had illness compatible with Lassa fever. Increasing international travel has resulted in importation of microbial agents not endemic to the United States, posing diagnostic challenges to health-care providers. In addition to routine evaluation, clinicians should consider both uncommon and common causes of fever (e.g., malaria) in persons arriving from Africa. Clinical histories should include careful assessment of travel to regions where uncommon diseases are endemic (e.g., for Lassa fever, Liberia, Nigeria, and Sierra Leone). Every effort should be made to expedite delivery of clinical specimens to appropriate diagnostic laboratories. The nonspecific presentation of Lassa fever and related viral infections that can cause viral hemorrhagic fever syndromes underscores the need for consistent application of infection-control practices. Suspected cases of Lassa fever or related infections should be reported immediately to hospital infection-control professionals and to state and local health departments for treatment recommendations and to facilitate implementation of infection-control precautions and tracing of contacts. Clinicians also should consult CDC's Special Pathogens Branch (telephone 404-639-1115), where specialized containment facilities exist to allow diagnostic confirmation by serologic, virologic, molecular, and pathology techniques. State health departments should notify DGMQ immediately of travel-related importations of suspected communicable diseases to ensure that prompt risk assessments, notifications, and appropriate containment measures are implemented for exposed travelers. Acknowledgments This report is based, in part, on contributions by A Chakarala, MD, M Gugnani, MD, G Gushue, MD, S Kindsfather, MD, S Shukla, MD, A Simonian, MD, T Sudhakar, MD, M Sullivan, L Vadakara, MD, R Wood, MD, Capital Health System, Trenton; J Blumenstock, A Monaco, New Jersey Dept of Health and Senior Svcs. L Mascola, MD, Los Angeles County Dept of Health Svcs, California. JL Hadler, MD, Connecticut Dept of Public Health. J Howell, DVM, Indiana State Dept of Health. D Weiss, MD, New York City Dept of Health and Mental Hygiene; B Ostrowsky, MD, Westchester County Dept of Health; G Johnson, MD, New York State Dept of Health. J Hoffman, Mecklenburg County Health Dept; K Dail, Div of Public Health, North Carolina Dept of Health and Human Svcs. D Hawkins, MS, Virginia Dept of Health. DM Nguyen, MD, S Schafer, MD, EIS officers, CDC. References
Figure 1  Return to top. Figure 2 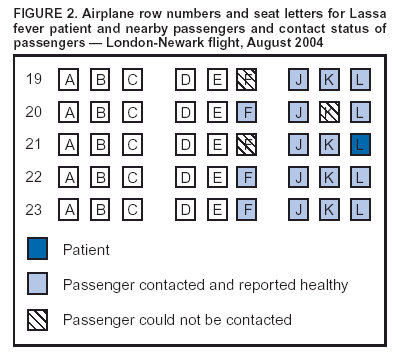 Return to top. Box  Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 9/30/2004 |
|||||||||
This page last reviewed 9/30/2004
|