 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Severe Acute Pneumonitis Among Deployed U.S. Military Personnel --- Southwest Asia, March--August 2003During March--August 2003, a total of 19 U.S. military personnel deployed in the Central Command (CENTCOM) area of responsibility had bilateral pneumonitis requiring intubation and mechanical ventilation (Figure); two patients died. This report summarizes the results of the U.S. Army's investigation of these cases and describes the ongoing investigation to determine the cause(s). Cases of rapidly progressive respiratory failure among former or current CENTCOM personnel should be reported to state health departments and to the Department of Defense (DoD). Of the 19 patients (median age: 25 years; range: 19--47 years), 18 were men; 12 were full-time active duty personnel, and seven were in the Reserve Component or National Guard (based in Arkansas, Illinois, Indiana, Kansas, Missouri, New Mexico, and North Dakota). Seventeen were in the Army, one was in the Navy, and one was in the Marine Corps; 11 were junior enlisted personnel, seven were noncommissioned officers, and one was an officer. Military specialties included combat arms (eight), engineering (three), transportation (two), signal corps (two), medical services (two), supply (one), and military police (one). Illness onset occurred a median of 81 days (range: 1--189 days) after arrival in the area of responsibility. Ten patients had evidence of elevated eosinophils in at least one of the following: peripheral blood (eight), bronchoalveolar lavage fluid (three), pulmonary tissue (one), or pleural fluid (one). Among the eight patients with peripheral eosinophilia, the maximum absolute number of eosinophils was 2,000--6,600 µ/L of blood (normal: <600 µ/L). The peripheral eosinophilia was detected a median of 6 days (range: 4--11 days) after illness onset. An interim case definition has been established. A confirmed case of severe acute pneumonitis with elevated eosinophils is defined as an illness occurring in a current or former member of the U.S. armed forces or a U.S. government employee deployed to the CENTCOM area of responsibility who had 1) bilateral pneumonitis (i.e., radiographically confirmed pulmonary infiltrates) that required mechanical ventilation and that did not result from a complication of another medical condition and 2) elevated pulmonary eosinophils (identified histologically, in bronchoalveolar lavage fluid [>5%] or in pleural fluid [>5%]). A probable case is defined as an illness in a person deployed to the CENTCOM area of responsibility who had bilateral pneumonitis requiring mechanical ventilation and the presence of peripheral eosinophilia (>600 µ/L blood absolute count). A suspect case is defined as an illness in a person deployed to the CENTCOM area of responsibility who had bilateral pneumonitis requiring mechanical ventilation only. As of September 8, four cases were confirmed, six were probable, and nine were suspect. Four patients had laboratory evidence of infection with a microbial agent. Streptococcus pneumoniae was isolated from sputum culture in one probable case. Three patients with suspect cases showed evidence of infection (S. pneumoniae based on urine antigen, Coxiella burnettii based on serology, and Acinetobacter baumannii from bronchoscopic culture). All patients were treated with broad-spectrum antibiotics, and six received corticosteroids, including two patients whose cases were confirmed and three whose cases were probable. The course of illness varied (median duration of intubation: 6 days; range: 2--35 days). For some patients, infiltrates and respiratory failure resolved rapidly (i.e., 2--3 days) with or without steroids, and other patients required longer periods of mechanical ventilation. All 17 surviving patients either have been placed on convalescent leave or have returned to duty. When they became ill, 13 patients were in Iraq, and six were in other countries (Kuwait [three], Djibouti [one], Qatar [one], and Uzbekistan [one]). Other than two patients from the same unit with suspect cases and with onset of illness 4 months apart, no apparent geographic or unit-level clustering has been identified. Of the 19 patients, 15 (79%) smoked cigarettes or cigars, including the 10 patients whose cases were either confirmed or probable. Nine of these 10 patients had begun smoking tobacco after deployment, compared with none of the nine patients whose cases were suspect. Two recent-onset smokers reported smoking non-U.S.-brand cigarettes. All troops in the CENTCOM area of responsibility have been exposed to heat, dust, and various amounts of environmental pollution (e.g., smoke). The U.S. Army is conducting a clinical and epidemiologic investigation to identify the cause(s) of this disease, including intensive testing of clinical material (i.e., blood, urine, bronchoalveolar lavage fluid, and acute and convalescent sera) to identify potential microbial pathogens and toxins. In addition, military personnel are interviewing patients systematically to identify any common exposures or practices. Environmental testing to identify potential toxins will be guided by clinical diagnostic and patient surveys. Initial data analysis suggests that medications, vaccines, and biologic weapons are not associated with the disease. Reported by: Operation Iraqi Freedom Severe Acute Pneumonitis Epidemiology Group, U.S. Army Medical Command. National Center for Infectious Diseases; National Center for Environmental Health, CDC. Editorial Note:The majority of cases of acute lower respiratory illness (LRI) among U.S. military personnel in Southwest Asia have been comparable clinically and have occurred at a rate similar to those in other military populations and settings (1). In contrast, the rapidly progressive LRI cases described in this report were life-threatening and required intensive medical care, including mechanical ventilation with high-end expiratory pressures. Although investigations are ongoing, preliminary findings suggest a subset of these cases are compatible with the diagnosis of acute eosinophilic pneumonia (AEP). AEP is an acute febrile illness without an identifiable infectious cause that is characterized by the rapid onset and progression of respiratory failure, diffuse bilateral infiltrates on chest radiographs, and elevated eosinophils in lung biopsy specimens or bronchoalveolar lavage fluid (2). Cigarette smoking (particularly of recent onset) is a risk factor for AEP (3--7), and some affected persons have experienced acute respiratory distress when exposed to cigarette smoke in a laboratory setting (5,6). The finding that nine of the 10 persons whose cases were severe and who had documented elevated eosinophils started smoking cigarettes after their deployment suggests the possibility of a toxin or allergen exposure; however, no single brand of cigarette or location of production has been implicated in this association. DoD has advised CENTCOM personnel that cigarette smoking, particularly the initiation of smoking, might be associated with the development of severe acute pneumonitis with elevated eosinophils. In 1997, two U.S. soldiers had rapidly progressive acute respiratory distress syndrome and elevated eosinophils shortly after returning from field training in the Mojave Desert in California (8). The occurrence of these cases in troops who were not deployed overseas suggests that exposures unique to Iraq (e.g., abandoned buildings, unexploded ordnance, and war-damaged vehicles or equipment) or to any of the countries in which the cases occurred (e.g., indigenous food, water, and materials) might not be necessary or sufficient for the development of this disease. No U.S.-based military personnel are known to have had severe acute pneumonitis with increased eosinophils during this period. However, the return of troops from Southwest Asia raises the possibility that U.S. health-care providers might be the first to observe members of this population who experience otherwise unexplained, acute respiratory failure. Clinicians should elicit the travel histories of patients with rapidly progressive respiratory failure of unknown etiology and report cases occurring among persons, particularly military personnel, who have returned recently from the CENTCOM area of responsibility to their state health department and to the U.S. Army Center for Health Promotion and Preventive Medicine, telephone 410-436-4655. References
Figure 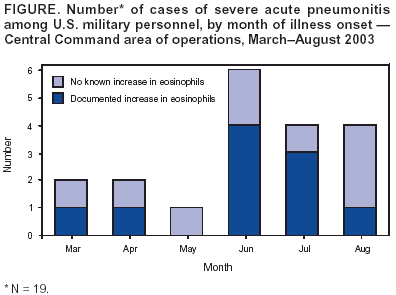 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 9/11/2003 |
|||||||||
This page last reviewed 9/11/2003
|