 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Respiratory Syncytial Virus Activity -- United States, 1996-97 SeasonRespiratory syncytial virus (RSV), a common cause of winter outbreaks of acute respiratory disease, results in an estimated 90,000 hospitalizations and 4500 deaths each year from lower respiratory tract disease in both infants and young children in the United States (1). Outbreaks occur annually throughout the country (2). RSV activity in the United States is monitored by the National Respiratory and Enteric Virus Surveillance System (NREVSS), a voluntary, laboratory-based system. This report summarizes trends in RSV from the NREVSS from July 1, 1990, through June 28, 1996, and presents provisional surveillance results for June 29-November 29, 1996. These data indicate onset of widespread RSV activity for the 1996-97 season. Since July 1, 1990, a total of 98 hospital-based and public health laboratories in 47 states have participated in the NREVSS and have reported weekly to CDC the number of specimens tested for RSV by the antigen-detection and virus-isolation methods and the number of positive results. Widespread RSV activity is defined by the NREVSS as the first of two consecutive weeks during which at least half of the participating laboratories report any RSV detections. This definition generally indicates a mean percentage of specimens positive by antigen detection in excess of 10%. During the previous six seasons, from July 1990 through June 1996, onset of widespread RSV activity began in November and continued for a mean of 22 weeks until April (Figure_1). In most parts of the 48 contiguous states, activity peaked each year in January or February; however, in the Southeast, activity peaked as early as November or December (3). For the reporting period June 29-November 29, 1996, a total of 75 laboratories in 45 states reported results of testing for RSV. Since the week ending November 22, more than half of the participating laboratories reported detections of RSV on a weekly basis, indicating onset of widespread RSV activity for the 1996-97 season. Reported by: National Respiratory and Enteric Virus Surveillance System collaborating laboratories. Respiratory and Enterovirus Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases, CDC. Editorial NoteEditorial Note: During the RSV season, health-care providers should consider the role of RSV as a cause of acute respiratory disease in both children and adults. Most severe manifestations of infection with RSV (e.g., pneumonia and bronchiolitis) occur in infants aged 2-6 months; however, children of any age with underlying cardiac or pulmonary disease or who are immunocompromised are at risk for serious complications from this infection. Because natural infection with RSV provides limited protective immunity, RSV causes repeated symptomatic infections throughout life. In adults, RSV usually causes upper respiratory tract manifestations but may cause lower respiratory tract disease -- especially in the elderly and in immunocompromised persons (4-6). Infection in immunocompromised persons can be associated with high death rates (6). RSV is a common, but preventable, cause of nosocomially acquired infection; the risk for nosocomial transmission increases during community outbreaks. Sources for nosocomially acquired infection include infected patients, staff, visitors, or contaminated fomites. Nosocomial outbreaks or transmission of RSV can be controlled with strict attention to contact-isolation procedures (7). In addition, chemotherapy with ribavirin may be considered for some patients (e.g., those at high risk for severe complications or who are seriously ill with this infection) (8); respiratory syncytial virus immune globulin intravenous (human) for high-risk patients was licensed for use in January 1996 (9). Vaccines for RSV are being developed, but none have been demonstrated to be safe and efficacious (10). References
Figure_1 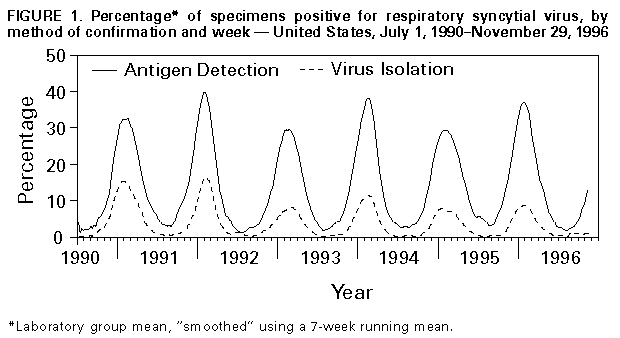 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|