At a glance
Methods from the Updated Recommendations on the Use of Chlorhexidine-Impregnated Dressings for Prevention of Intravascular Catheter-Related Infections (2017).
Methods
CDC developed the following key question using the PICO (Patient, Intervention, Comparator, Outcome) format to guide the search of published literature on C-I dressings in adults (defined as patients aged 18 years and older) and children (defined as patients younger than 18 years).5
- Does use of C-I dressings, compared with use of standard dressings, affect the risk of intravascular infections associated with short-term, non-tunneled central venous catheters in adults and children?
CDC conducted a systematic review of the best available evidence on C-I dressings. CDC then used a modification of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method to assess the quality of the available evidence, to determine the strength of recommendations, and to show the relation between evidence and recommendations.6,7,20
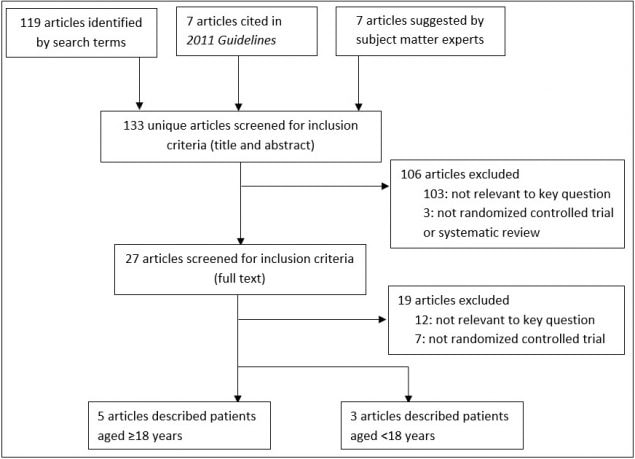
Two reviewers (Dasti, Overholt) systematically searched articles indexed in MEDLINE and the Cochrane Library for articles published through March 6, 2017 (Appendix Table 1, Appendix Table 2, and Appendix Table 3). Two reviewers (Overholt, Stone) screened article titles and abstracts and retrieved full text articles if they were:
- relevant to the key question;
- randomized controlled trials, systematic reviews, or meta-analyses;
- written in English; and
- available as full-text studies (excluding published meeting abstracts).
These reviewers also reviewed the full-text articles and excluded articles that were:
- conducted in dialysis settings, and
- not RCTs (Figure 1).
Disagreements were resolved by discussion.
For studies that met the inclusion criteria, two reviewers (Overholt, Stone) extracted data on: the study author, year, study design, objective, population, intervention, outcome definitions, intervention and control events, hazard ratios, confidence intervals, and p-values. Reviewers contacted authors of selected studies to confirm the skin antisepsis agent and use of daily chlorhexidine bathing if these details were not reported in the article. They extracted data as originally presented in the articles and resolved discrepancies through discussion. Two reviewers (Overholt, Stone) assessed the risk of bias for each RCT using an index developed by the University of Pennsylvania Health System's Center for Evidence-Based Practice, as had been used for recent CDC and HICPAC guidelines (Appendix Table 8 and Appendix Table 9).
Figure 1: Yield of Systematic Search of Articles Published January 2010–March 6, 2017
The guideline writing group (comprised experts in infection control and evidence-based guideline development; listed as authors) reviewed the findings from the evidence review and formulated recommendations based on the balance of benefits and harms of C-I dressings when used for preventing infections associated with short-term, non-tunneled catheters. The strength of each recommendation was based on the categorization scheme used for previous CDC healthcare infection control guidelines (Table 1). The writing group did not consider the following issues when formulating recommendations: cost or cost-effectiveness of C-I dressings in healthcare facilities with different underlying rates of catheter use or catheter-related infections; dressing preferences of providers or health systems; provider opinions about ease of dressing application, removal, or inspection for complications; or the impact of dressing use on other aspects of catheter care (e.g., frequency of dressing change, compatibility with catheter materials). Other sources describe additional details of the guideline development process.21,22
