At a glance
These instructions are in accordance with CDC regulations and are for the use of panel physicians evaluating persons applying for immigrant or refugee status, as well as certain non-immigrants who are required to have an overseas medical examination.
Preface
The medical screening for syphilis among persons overseas applying for U.S. immigrant or refugee status, as well as non-immigrants who are required to have an overseas medical examination, hereafter referred to as applicants, is an essential component of the immigration process. Because syphilis may be challenging to diagnose and treat, these Technical Instructions provide panel physicians a method for recording findings from the immigration medical examination, as well as provide additional guidance for the panel physician in classifying applicants.
The instructions in this document supersede all previous Technical Instructions, Updates to the Technical Instructions, memoranda and letters to panel physicians, and memoranda and letters to international refugee resettlement organizations. These Technical Instructions are to be followed for syphilis screening and treatment among all applicants and are effective as of September 3, 2021.
Visit the Technical Instructions for Panel Physicians webpage for more information about the medical examination for applicants for U.S. immigration.
Key Concepts
- All applicants aged 18 years to those aged less than 45 years must be tested for evidence of syphilis.
- Applicants aged less than 18 years or 45 years or greater must be tested if there is reason to suspect infection with syphilis.
- Nontreponemal and treponemal laboratory tests must be ordered by the panel physician at the time of the immigration medical examination and these tests must be performed on the same blood sample. Tests performed elsewhere, or prior to the panel physician's examination of the applicant, are not acceptable.
- The traditional algorithm is the required approach for syphilis laboratory testing.
- External genital and rectal examinations for staging syphilis must only be performed AFTER laboratory confirmation of diagnosis and a chaperone must be present during these examinations.
Syphilis Screening
Syphilis is a sexually transmitted, systemic disease caused by the bacterium Treponema pallidum subspecies pallidum. The disease has often been called "the great imitator" because of its wide variety of signs and symptoms, with different stages having different clinical manifestations. There are three infectious stages (primary, secondary, and early latent disease) and two noninfectious stages (late latent and tertiary disease). Untreated syphilis can progress and lead to serious long-term sequelae and, rarely, death.
Medical History
Obtaining the applicant's medical history must include inquiring about prior history and treatment for syphilis; a sexual partner with syphilis; or any history of painless sores on the genitals, anus, or mouth or a rash on the body, especially on the palms of the hands or soles of the feet.
Physical Examination
If the history or serologic tests are suggestive, a physical examination may be warranted that should include an evaluation for oral signs of syphilis, including mouth sores (chancres) or mucus patches, or rashes on the body, particularly on the palms of the hands or soles of the feet (a characteristic of syphilis infection that is unusual in other conditions). An external genital examination is not required and must not be performed unless the applicant has laboratory confirmation of diagnosis (positive nontreponemal and treponemal test results); these applicants should undergo an evaluation for external genital, anal, or perianal sores (chancres) or other lesions (i.e., condyloma lata) to help determine the stage of syphilis. A gown or sheet must be provided to allow privacy and a chaperone MUST be present, regardless of the applicant's gender, if external genital and rectal exams are needed. The chaperone must be a staff member of the gender the patient feels most comfortable with and not a family member. However, family members may be present for the exam if the applicant requests. Sores consistent with syphilis are typically painless, indurated, clean-based genital, rectal or oral ulcers. Regional lymphadenopathy may be present in primary or secondary syphilis. Applicants with reactive serologic tests consistent with syphilis should be asked specifically about and evaluated for clinical symptoms or signs suggestive of neurosyphilis (e.g., cranial nerve dysfunction, meningitis, stroke, acute or chronic altered mental status, loss of vibration sense, and auditory or ophthalmic abnormalities). Neurosyphilis can occur at any stage of infection.
Histories or clinical signs suggesting syphilis in children must be properly evaluated with serologic testing. Physical examination findings of congenital syphilis in infants may include nonimmune hydrops, jaundice, hepatosplenomegaly, rhinitis, skin rash, and pseudoparalysis of an extremity. In older children, signs of untreated congenital infection may include interstitial keratitis (5-20 years of age), eighth cranial nerve deafness (10-40 years of age), Hutchinson teeth (peg-shaped, notched central incisors), anterior bowing of the shins, frontal bossing, mulberry molars, saddle nose, rhagades (linear scars around the mouth), and Clutton's joints (symmetric, painless swelling of the knees).
Laboratory Testing
Although various laboratory testing approaches exist for syphilis screening, CDC requires panel physicians to use the traditional testing algorithm starting with a nontreponemal test rather than a reverse testing algorithm at this time. All tests used by panel physicians must be conducted by appropriately trained technologists following standard operating procedures in a qualified laboratory that is enrolled in an external quality assurance program (e.g., proficiency testing) program.
A nontreponemal test (i.e., Venereal Disease Research Laboratory [VDRL] or Rapid Plasma Reagin [RPR]) should first be used for screening and positive or reactive results must have a quantitative titer result reported (e.g., 1:4) and documented on the Medical History and Physical Examination Worksheet (DS-3026) or the 712 Syphilis Test exam in the eMedical USA system. Positive or reactive results on nontreponemal tests must be confirmed using a treponemal test on the same blood sample and testing must be performed in this order. The following tests are allowed to be used for treponemal testing: T. pallidum passive particle agglutination (TP-PA) assay, Treponema pallidum haemagglutination (TPHA) test, enzyme immunoassays (EIAs), chemiluminescence immunoassays (CIAs), fluorescent treponemal antibody absorbed (FTA-ABS) tests, immunoblots (use products of this test that are either approved by the U.S. Food and Drug Administration [FDA] or recommended by the World Health Organization [WHO]), or rapid treponemal assays (use products of this test that are either FDA-approved or WHO-recommended).
Syphilis tests must be performed at the time of the immigration medical examination and at the laboratory stated in the panel physician agreement. Tests performed elsewhere, or prior to the panel physician's examination of the applicant, are not acceptable.
Children with history of physical stigmata suggesting syphilis infection must have serologic testing for syphilis. Older infants (aged ≥1 month) and children who are identified as having reactive serologic tests for syphilis should have maternal serology and records reviewed to assess whether they have congenital or acquired syphilis and whether they have been previously treated. Any child at risk for untreated congenital syphilis should be treated, and recommended to have a full evaluation, which may include CSF analysis, complete blood count and differential, and long-bone radiographs, depending upon previous documented evaluation. Dark field microscopic examination of suspicious lesions or body fluids (e.g., nasal discharge) also should be performed. Panel physicians should refer to CDC's Sexually Transmitted Infections (STI) Treatment Guidelines for specific guidance on testing in children.
All applicants diagnosed with syphilis should be advised to be tested for other STIs, including chlamydia, gonorrhea, and HIV. The consent for HIV testing should include the following:
- Applicants understand they do not have to be tested for HIV.
- Applicants understand that if they would like to be tested for HIV, they do not have to be tested for HIV by a panel physician.
- Applicants understand that panel physicians must include the test results on the paperwork they complete.
If the applicant consents, panel physicians should perform HIV testing.
Syphilis Screening Results and Travel Clearance
- Applicants with a positive (or reactive) nontreponemal test (i.e., RPR or VDRL) and a negative (or nonreactive) treponemal-specific test are No Class for syphilis.
- Applicants with a positive (or reactive) nontreponemal test (i.e., RPR or VDRL) and a positive (or reactive) treponemal test are Class A for syphilis and will remain Class A until treated.
- After completing treatment, applicants are classified as Class B.
- The evaluation is complete when the required aspects of the medical examination have been completed and the applicant is assigned a syphilis classification.
- Travel clearances for syphilis are valid for the same length of time as the applicant's tuberculosis screening evaluation.
It is important that syphilis be correctly diagnosed. Correct diagnosis of syphilis will ensure that affected applicants receive appropriate treatment minimizing long-term sequelae and reducing further spread of the disease. False-positive nontreponemal test results can be associated with multiple medical conditions and factors unrelated to syphilis, including other infections (e.g., HIV, tuberculosis, hepatitis, malaria), autoimmune conditions (e.g., systemic lupus, rheumatoid arthritis), vaccinations (e.g., smallpox, MMR), injection drug use, pregnancy, and older age. Therefore, persons with a positive (or reactive) nontreponemal test must always receive a treponemal test to confirm the syphilis diagnosis. Treponemal tests might be positive (or reactive) with serum from patients with other infectious and noninfectious diseases, conditions, Yaws (T. pallidum subspecies pertenue), Pinta (T. carateum), Bejel (T. pallidum subspecies endemicum) or other treponemal diseases.
Panel physicians must refer to CDC's STI Treatment Guidelines for more details regarding interpretation of nontreponemal test titers for applicants with a positive (or reactive) treponemal test and documented history of prior syphilis treatment. Only nontreponemal test titers from the same assay type can be compared directly. Official documentation of treatment history and previous laboratory results should be included with the applicant's exam packet or uploaded into eMedical USA system.
Figure 1. Syphilis screening using the traditional algorithm for all applicants aged 18 years to those aged less than 45 years*
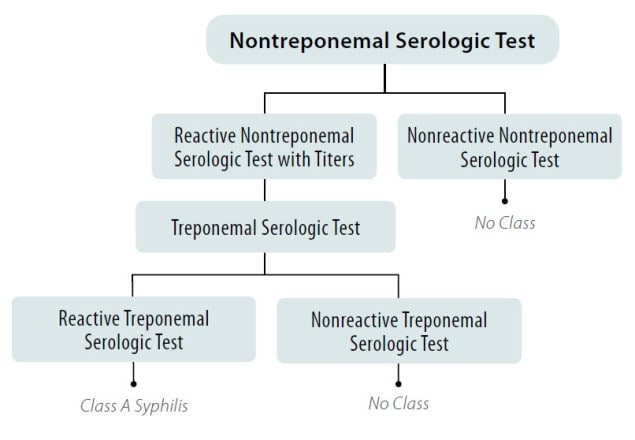
*And any applicant aged less than 18 years or 45 years of age or greater suspected of having syphilis infection
| Treponemal Test Results | ||
|---|---|---|
| Nontreponemal Test Results | Reactive | Non-Reactive |
| Reactive ≥ 1:8 |
|
|
| Reactive < 1:8 |
|
|
| Non-reactive |
|
|
Syphilis Treatment
Panel physicians must treat syphilis according to CDC's STI Treatment Guidelines, which are periodically updated.
- Details of testing and treatment must be provided on the Medical History and Physical Examination Worksheet (DS-3026) or the 712 Syphilis Test exam in the eMedical USA system.
- Benzathine penicillin G (BPG) is the preferred treatment regimen for syphilis. Although alternative regimens are known to be effective, applicants should be informed that BPG is the first-line recommendations in U.S. and WHO STI management guidelines.
- No proven alternatives to injectable penicillin regimens are available for treating neurosyphilis, congenital syphilis, or syphilis in pregnant women. Therefore, infected applicants with a history of penicillin allergy who have neurosyphilis or congenital syphilis, or who are pregnant are advised to be desensitized and treated with penicillin if appropriate facilities are available.
Treatment – Post Evaluation
- Adult applicants treated for syphilis should be informed by panel physicians that they will need follow-up care for clinical and serologic re-evaluation in 6 months (3 months if HIV positive and treated for primary or secondary syphilis).
- Children should receive follow-up care 2-3 months after treatment; this follow-up does not affect the examination validity period.
Waivers
Note:
A provision allows applicants undergoing treatment for syphilis to apply for a Class A waiver.
Waivers will become unnecessary after completion of treatment, as the applicant will be classified as Class B for syphilis.
A provision allows applicants with a Class A physical disorder to petition for a Class A waiver. If the applicant would like to pursue a waiver, an Application for Waiver of Grounds of Inadmissibility Form (I-601 or I-602 for immigrants or refugees, respectively) must be completed. These waivers are submitted to the Department of Homeland Security (DHS), U.S. Citizenship and Immigration Services (USCIS) on an individual basis. The CDC Division of Global Migration Health (DGMH) also reviews the waivers and supporting medical examination to provide an opinion regarding the case to the requesting entity (Department of State or DHS, USCIS). DGMH’s review of the waiver and supporting medical examination documentation is to ensure that the applicant has been classified properly and that an appropriate U.S. healthcare provider is identified for the applicant. DHS, USCIS has the final authority to adjudicate the waiver request.
Documentation
Note:
All medical documentation, including any laboratory reports, must be included with the required DS forms or the 712 Syphilis Test exam in the eMedical USA system.
Information recorded on the DS Forms or in the eMedical USA system should be in English and typed.
Applicants with Class A syphilis must be reported to the U.S. Embassy upon detection by sending all required medical documentation by courier or other secure means.
Department of State (DOS) forms Report of Medical Examination by Panel Physician (DS-2054), Vaccination Documentation Worksheet (DS-3025), Medical History and Physical Examination Worksheet (DS-3026), and Tuberculosis Worksheet (DS-3030) must be completed in their entirety and included in the applicant's travel packet, or within the eMedical USA system, specifically the 712 Syphilis Test exam. This includes assigning a syphilis classification (Class A or Class B) on the DS-2054 or the appropriate section in the eMedical USA system and attaching laboratory reports. Incomplete documentation may result in refusal to grant a visa or designation of medical hold status at arrival to U.S. ports of entry.
For applicants requiring syphilis treatment prior to U.S. immigration, the panel physician is required to document the following on the DS-3026 or in the 712 Syphilis Test exam in the eMedical USA system:
- Results and dates of treponemal and non-treponemal tests, including titers for nontreponemal tests. Type of test used should be noted
- Stage of syphilis based on history and clinical findings
- Drug regimen received (including doses, dosage units, and administration routes of all medications), start date, completion date, and any periods of interruption.
- Clinical course, if applicable, such as clinical improvement or lack of improvement during and after treatment for primary or secondary syphilis, including resolution of symptoms and signs and any drug reactions.
Glossary of Abbreviations
Acronym
Full Phrase
CDC
Centers for Disease Control and Prevention, United States
CIA
Chemiluminescence immunoassay
CSF
Cerebral spinal fluid
DGMH
Division of Global Migration Health
DHS
Department of Homeland Security
DOS
Department of State
EIA
Enzyme immunoassay
FTA-ABS
Fluorescent treponemal antibody absorbed
HIV
Human immunodeficiency virus
RPR
Rapid plasma reagin
STI
Sexually transmitted infection
TP-PA
T. pallidum passive particle agglutination assay
USCIS
United States Citizenship and Immigration Services
U.S. FDA
U.S. Food and Drug Administration
VDRL
Venereal Disease Research Laboratory
WHO
World Health Organization
