At a glance
- Effectively responding to pandemics depends on having accurate laboratory information.
- This concern led CDC to develop sustainable laboratory networks worldwide, enhancing countries' abilities to rapidly identify and eliminate disease threats.
- These initiatives enhance outbreak responses and prepare countries for future health emergencies, strengthening global health security through advanced laboratory technologies and training.
CDC strengthens laboratory networks to prepare for pandemics

Pandemics, like COVID-19, cannot be stopped effectively without critical information from laboratories. Laboratories help confirm the presence of disease, pinpoint the cause of illness, and guide the right response to outbreaks. CDC plays a leading role in developing sustainable laboratory networks that can safely, accurately, and rapidly identify disease threats so they can be eliminated at the source. CDC works with countries to help improve their ability to detect disease outbreaks by providing access to cutting-edge technology, expanding laboratory networks, and bringing environmentally safe waste management solutions to improve efficiency and streamline coordination across all levels of the healthcare system.
Through collaboration with countries such as Thailand, Democratic Republic of Congo (DRC), and Nigeria, countries are better able to perform diagnostic tests and help scientists prepare for future public health emergencies more quickly.
Thailand
Strengthening outbreak response with next-generation sequencing

The recent COVID-19 global pandemic highlighted the need to rapidly detect and respond to potential outbreaks. The most common approaches to identifying pathogens use tools and technology, such as PCR-based methods, to identify and compare the traits of known and unknown pathogens. However, next-generation sequencing (NGS) technology provides a higher power to detect genetic material with speed and scalability. NGS has allowed laboratories to perform a variety of applications and analyze systems at a level never achieved before. Genomic sequencing finds the order of the chemical building blocks, or bases, that form an RNA molecule which allows the study of how the pathogen is changing over time.
During 2021-2022, CDC developed a pilot program, Global Network for Genome Sequencing of SARS-CoV-2 (the virus that causes COVID-19) to establish NGS capacity by strengthening the ability to test for pathogens in select countries. Because staff were already trained and the required equipment was in place at the start of the pandemic, pilot program countries like Thailand were able to quickly pivot to sequencing SARS-CoV-2. In December 2021, CDC's Dr. Pongpun Sawatwong and his team confirmed the first case of the Omicron variant in Thailand using NGS.
CDC experts within the Division of Global Health Protection (DGHP) and DGHP Thailand Laboratory team worked together to leverage existing capacity for pathogen testing during the COVID-19 response to identify variants as they emerged, allowing more time for the health system to respond and save lives. Since the COVID-19 pilot program, Dr. Sawatwong and his team have been involved with NGS of bacterial pathogens. CDC is dedicated to supporting countries in the implementation and use of advanced laboratory technology and training and working alongside partners to better equip countries to identify, respond, and prepare for emerging public health threats.
Democratic Republic of Congo (DRC)
Establishing a strong tiered laboratory network
Public health systems with functioning diagnostic laboratories at multiple levels offer several advantages when compared to tiered laboratory systems with centralized national testing. Usually, these laboratories reduce the time between sample collection and testing, and can identify pathogens more rapidly. Tiered laboratory networks include laboratories at different levels (district, provincial, regional, and national) with increasing complexity and capacity for testing at each level. Tiered laboratory networks are critical to strengthening public health laboratory systems and increasing the laboratory capacity to test for pathogens in a timely manner.
CDC laboratory experts provided guidance on improving both testing capabilities and general laboratory upkeep to assist the DRC Ministry of Health and ICAP at Columbia University in developing tiered laboratory networks. DRC's new laboratory within the Lubumbashi Provincial Laboratory allowed diagnostic testing for numerous pathogens instead of shipping samples to the national laboratory in Kinshasa for testing. To support community laboratory services within the Mbandaka Region of DRC, Mbandaka Provincial Laboratory contributed to facility repairs to improve the water source and quality, electrical wiring, and laboratory work areas to expand its testing capabilities in the future. CDC also funded procurements such as cold chain equipment, autoclaves and solar panels for electrical power and back-up for some laboratory sections.
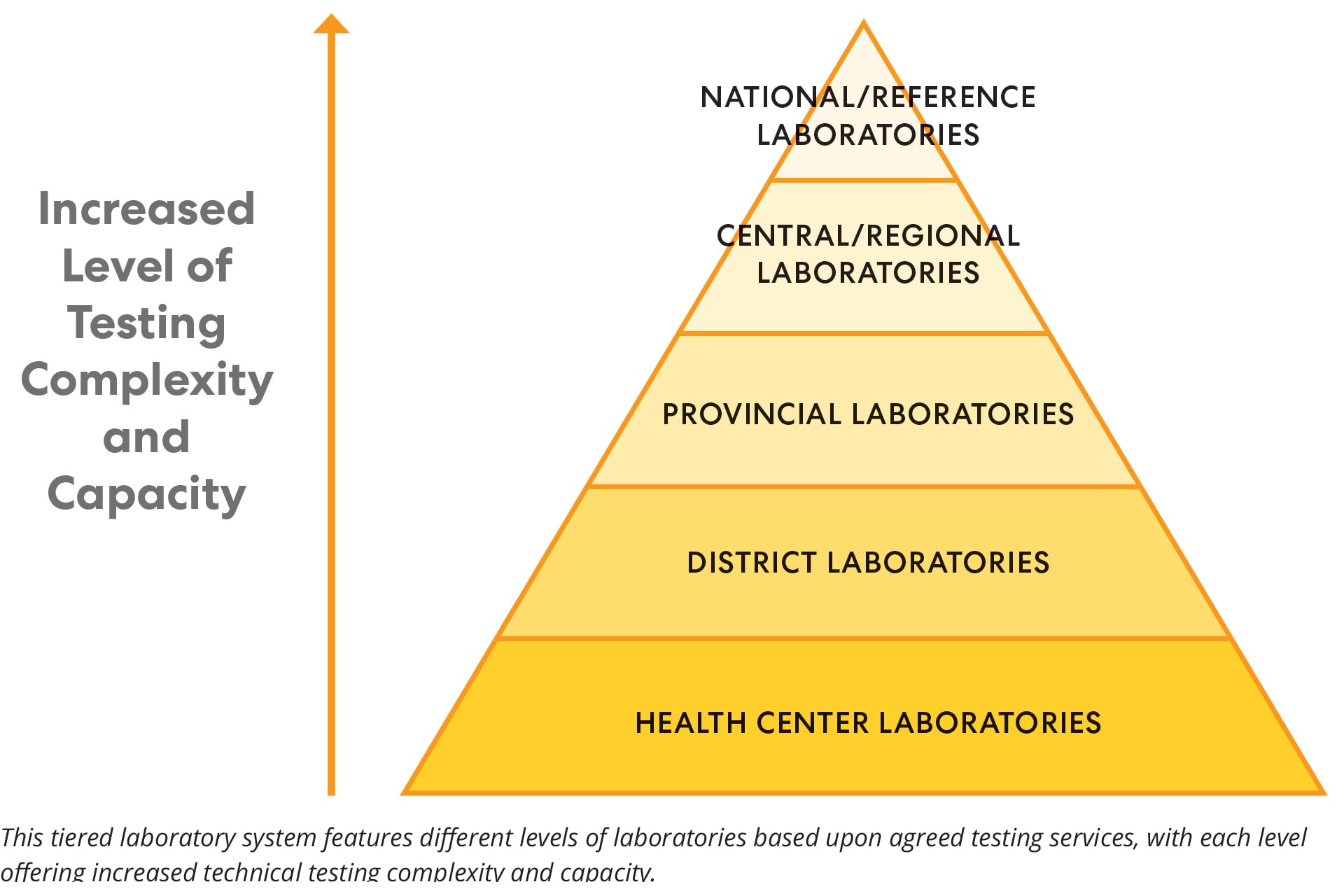
With CDC's expertise in creating strong tiered networks, countries such as DRC can improve their laboratories and reduce the number of samples that require long-distance transportation. When samples require more sophisticated testing methods, CDC can also provide technical advice on the safe and efficient transportation of samples to appropriate facilities for testing. When smaller laboratories are built or updated, countries are not forced to rely upon national laboratories alone or outdated technology for their testing needs. This results in more efficient and effective use of laboratory resources and faster responses to public health emergencies.
Nigeria
Developing biowaste management systems
After laboratories test samples for pathogens, the samples and other waste generated in the testing process, known as biowaste, need to be safely discarded. Improper waste disposal from laboratories can harm the laboratory, environment, and people. Biowaste management systems provide protection to laboratorians and the surrounding environment.
In 2022, to address needs identified during a laboratory assessment, CDC collaborated with the Defense Threat Reduction Agency (DTRA) and Nigeria CDC to establish a modern and sustainable biowaste management system at Nigeria CDC after laboratory facility assessments identified the need. A modern autoclave system, which sterilizes laboratory equipment, supplies, and waste using high-temperature steam, was placed in a Nigeria CDC laboratory to prevent the growth of microorganisms on surfaces, improving safety and reducing the threat of pathogen exposure.
Given the challenges with waste that laboratories generate, experts determined the need for a biomedical waste incinerator. In August 2022, a standard high-temperature incinerator was installed at the National Reference Laboratory (NRL) in Abuja. The incinerator burns materials, compounds generated during testing, and other infectious waste materials (solid and liquid) from the NRL and other laboratories across the country that have been mapped to the site for centrally coordinated laboratory waste management. The installed incinerator is coupled to a Pollution Control System (PCS) to remove gaseous and particulate emissions from the incinerator that may be harmful to the environment.
Nigeria CDC now has a country-owned and sustainable system for the safe management of biological waste from laboratories and clinical facilities that it supports. This closes a major gap identified in laboratory facility assessments. DGHP played an important role in the process by leveraging expertise from across CDC to support Nigeria in improving its laboratory function and increasing the safety of laboratorians and the community.
Increased access to emerging technologies prepare countries for future outbreaks
CDC continues to strengthen laboratory systems by creating and enhancing global networks that can conduct testing, analyze findings, manage data, and protect against biosafety and biosecurity threats. By collaborating with countries and partners around the world, countries have the needed laboratory information to identify and recognize threats early, implement stronger testing approaches, and prepare for future outbreaks.
