At a glance
To provide further insight into the discrepancy between intent and coverage, the authors examined attitudes and beliefs about the bivalent booster vaccine from two sources. Findings in this report may assist in planning for implementation of new COVID-19 vaccine recommendations.
Summary
In September 2022, the CDC recommended an updated, bivalent booster vaccine to all adults with a completed COVID-19 primary vaccination series1. The bivalent vaccine was introduced to better protect against Omicron BA.4 and BA.5 subvariants in addition to the original COVID-19 strain2. Interviews conducted on behalf of the CDC during November–December 2022 show that while most vaccinated adults were open to receiving a booster vaccination, only 27.1% had received one by the end of 20223. To provide further insight into the discrepancy between intent and coverage, we examined attitudes and beliefs about the bivalent booster vaccine from two sources: omnibus surveys sponsored by the CDC and conducted by IpsosA and NORCB in March and April of 2023, and the Household Pulse Survey (HPS)C conducted by the U.S. Census Bureau during the same months. According to the omnibus surveys, the most cited concerns among those who had not yet, but were open to receiving the bivalent booster, were side effects of the vaccine and being too busy or keeping forgetting about the vaccine. Findings from the HPS similarly show that many adults did not have a specific reason why they had not received a bivalent vaccine; they planned on getting one and just had not yet done so. The FDA recommended an update to the COVID-19 vaccine for fall 2023, advising manufacturers to target the XBB lineage of the Omicron variant4. Findings in this report on attitudes towards the 2022 updated (bivalent) COVID-19 vaccine may assist in planning for implementation of new COVID-19 vaccine recommendations.
Results from the Omnibus surveys
Figure 1: Vaccination status and intent to receive bivalent COVID-19 vaccine, by demographics, among adults with completed primary series (Omnibus survey, March-April 2023)
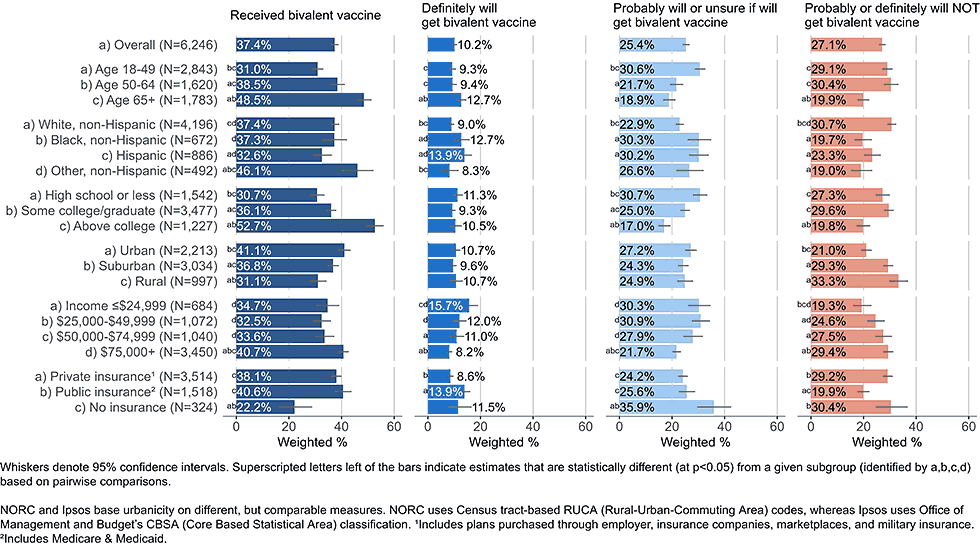
Overall, 35.6% of respondents were open to receiving a bivalent booster vaccine, stating they would either definitely get the updated vaccine (10.2%) or would probably get it or were unsure (25.4%)D. Low bivalent vaccine uptake was not always indicative of low intent. Some racial and ethnic groups and income groups showed lower coverage but not lower intent than their counterparts. Adults ages 18-49 years and uninsured adults had lower coverage than adults over the age of 50 or insured adults, but among those who had not received a bivalent vaccine both of these groups were more open to vaccination than their counterparts.
Importantly, even among groups with higher bivalent coverage, a large percentage reported not having received the vaccine, but being open to getting it, including among adults ≥65 years. Coverage of 70% or higher could be achieved in most demographic subgroups if all who are open to bivalent boosters were to receive them.
Figure 2: Concerns regarding bivalent COVID-19 vaccines, by intent to receive bivalent vaccine, among adults with a completed primary series (Omnibus survey, March-April 2023)
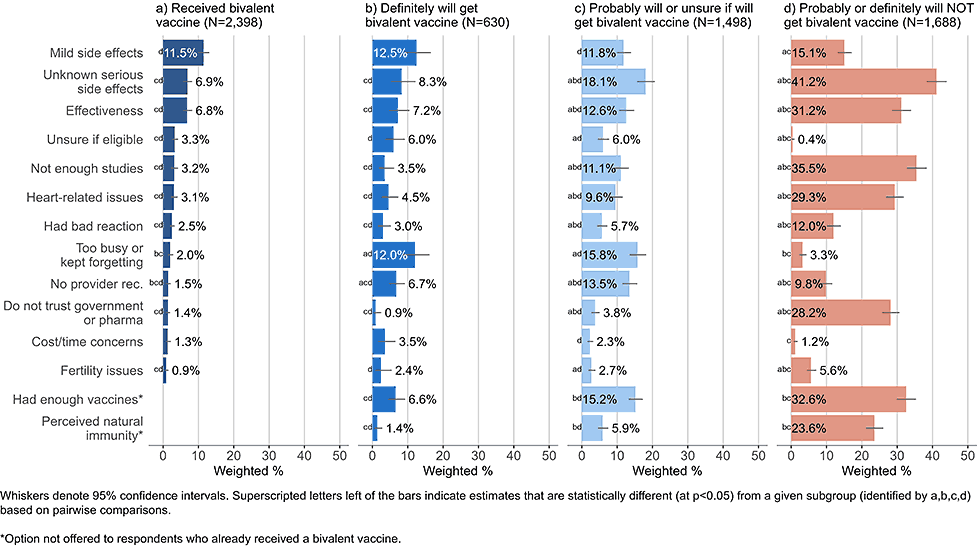
- Respondents who said they probably will get or are unsure about getting a bivalent vaccine and respondents who probably or definitely will not get a bivalent vaccine were most concerned with unknown serious side effects (18.1% and 41.2%, respectively).
- Adults who stated they probably or definitely will not get a bivalent vaccine also commonly reported concerns about vaccine effectiveness, potential cardiovascular side effects, wanting to wait for more studies, mistrust of government and pharmaceutical industry, and not needing or already having had enough vaccines; these concerns were much less commonly reported by those already vaccinated or open to vaccination (definitely will get, probably will get, or unsure about getting a bivalent vaccine).
- Adults open to vaccination were more likely than others to state they did not get a bivalent vaccine because they were too busy or kept forgetting.
- 5% of those who said they probably would get a booster or are unsure cited not receiving a recommendation from a healthcare provider as a concern.
- Practical barriers, such as cost, not knowing where to get vaccinated, or not knowing if eligible, were infrequently cited among all intent groups.
Concerns about the bivalent booster vaccine among population open to vaccination by subgroups
The following figures present concerns about bivalent booster vaccine by demographic subgroups, focusing on those who have not yet, but are open to receiving the bivalent booster vaccine (respondents who have not yet received, but report they will either definitely or probably get or are unsure about getting a bivalent booster dose).
Figure 3: Concerns regarding bivalent COVID-19 vaccines, by age, among adults with completed primary series who are open* to receiving a bivalent vaccine (Omnibus survey, March-April 2023)
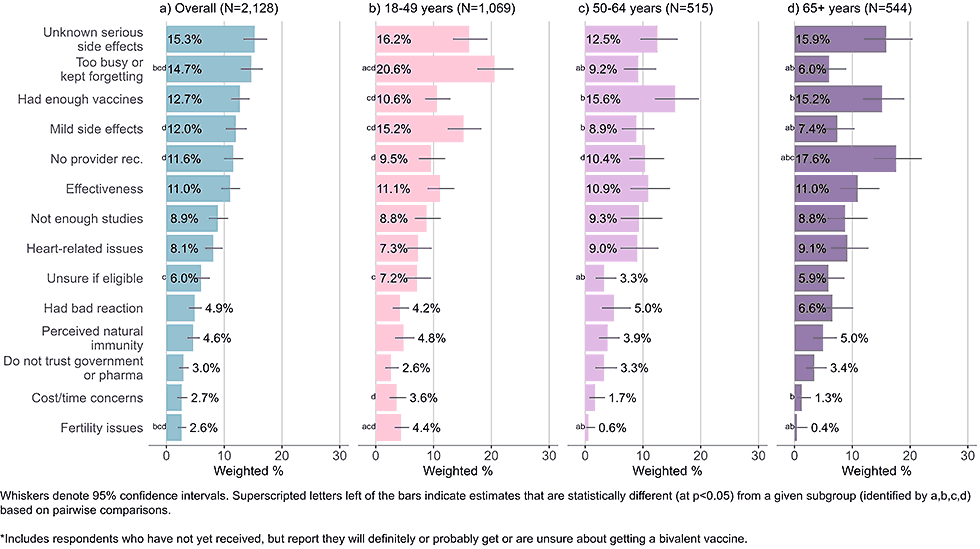
- Adults ages 65 years and older reported not having received a recommendation from their healthcare provider as a main concern. They were also more likely than adults under 50 years to say they had received enough COVID-19 doses.
- Younger adults were most likely to indicate they were too busy or kept forgetting to get the vaccine. Mild, short term side effects were another common and overrepresented concern among young adults.
- All age groups listed unknown, serious side effects as one of their main concerns.
Figure 4: Concerns regarding bivalent COVID-19 vaccines, by race and ethnicity, among adults with completed primary series who are open* to receiving a bivalent vaccine (Omnibus survey, March-April 2023)
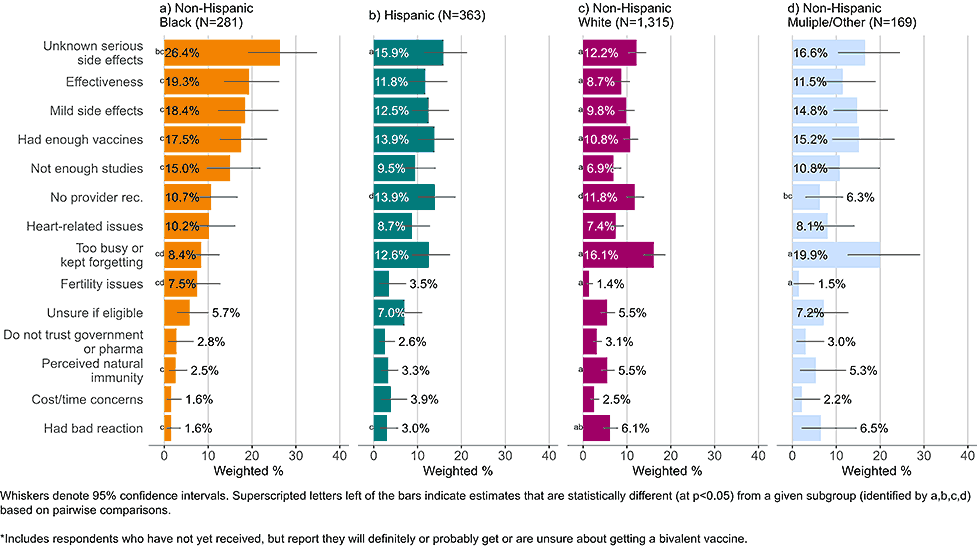
- Black, non-Hispanic respondents generally had more concerns regarding the bivalent vaccine than their counterparts. They commonly listed unknown, serious side effects, known, short-term mild side effects, effectiveness, and wanting to wait for more studies as concerns.
- A plurality of non-Hispanic White adults did not list a particular concern with the bivalent vaccine but instead stated they were too busy or kept forgetting to get the bivalent vaccine.
Figure 5: Concerns regarding bivalent COVID-19 vaccines, by income, among adults with completed primary series who are open* to receiving a bivalent vaccine (Omnibus survey, March-April 2023)
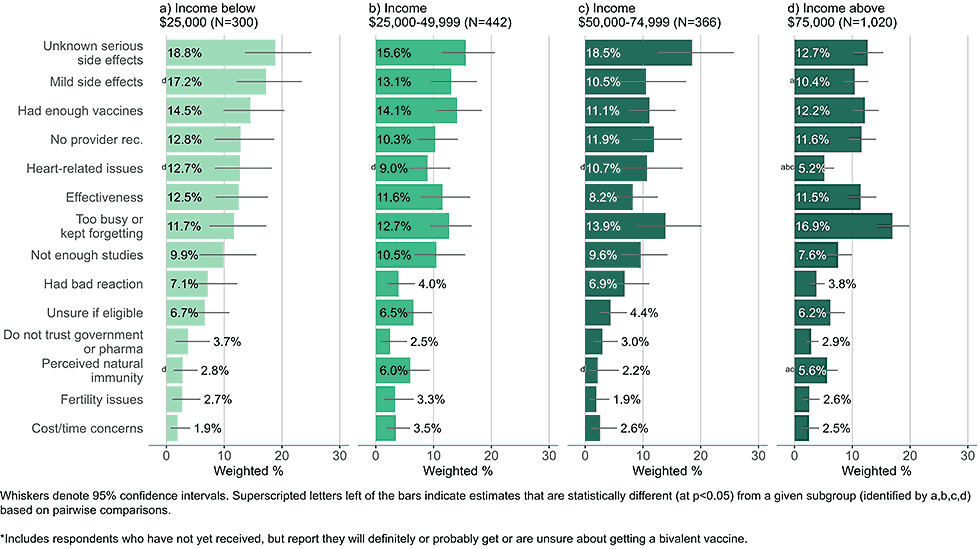
- Low-income adults were more likely than high income adults to be concerned about unknown, serious side effects, heart related side effects, and mild side effects.
- Concerns about cost were infrequently reported, even among those in the lowest income groups.
Figure 6: Concerns regarding bivalent COVID-19 vaccines, by insurance status, among adults with completed primary series who are open* to receiving a bivalent vaccine (Omnibus survey, March-April 2023)
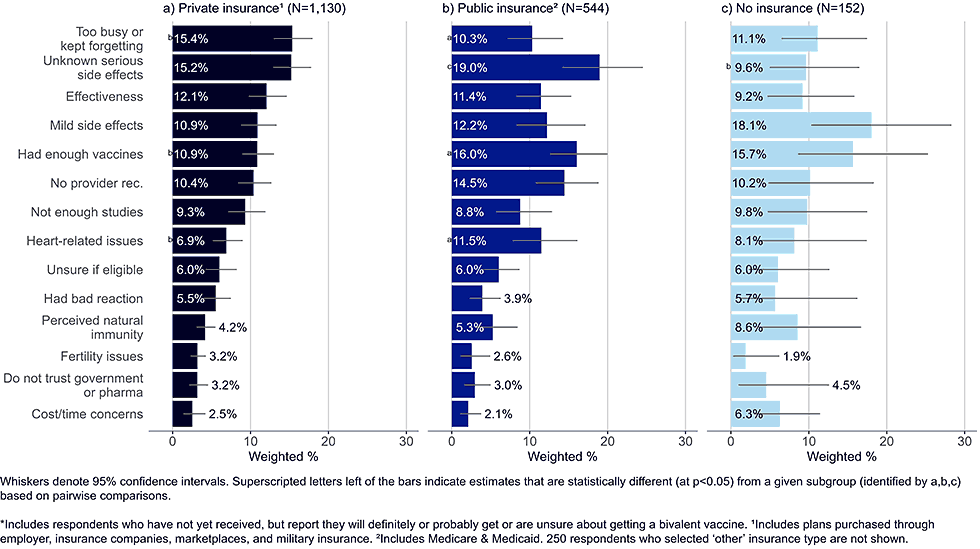
- Publicly insured adults were more likely to state they had enough vaccines and were more concerned with heart-related issues than adults with private insurance.
- While the sample size of uninsured adults was small, top concerns reported by this group included mild side effects and that they have had enough vaccines.
Results from household pulse surveys
To supplement our analysis, we analyzed data from the Household Pulse Survey (HPS) on reasons reported by adults with at least one COVID-19 vaccination but no bivalent booster for not having yet received a bivalent boosterE.
Figure 8: Reasons for not yet having received a bivalent COVID-19 vaccine by age, among adults with 1+ dose of COVID-19 vaccine (Household Pulse Survey, March-April 2023)
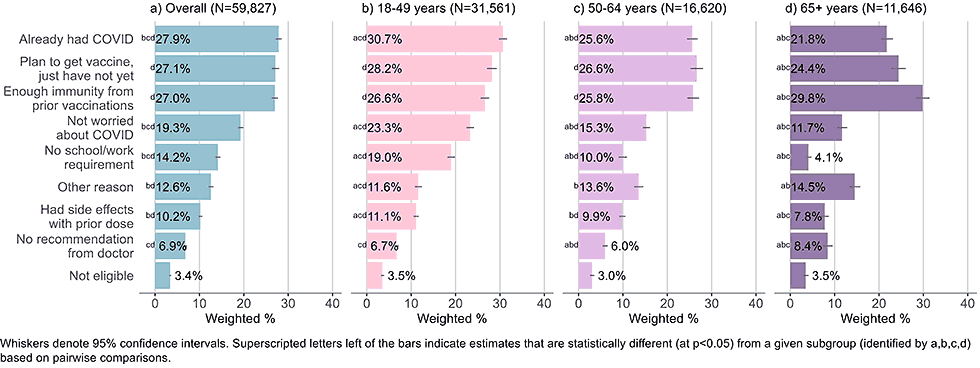
- Adults ages 65 and older were most likely to indicate they had enough immunity from prior vaccines, adults 18-49 were most likely to indicate they already had COVID-19, and adults 18-49 and 50-64 were equally likely to select all three main choices.
- The youngest cohort was more likely than adults age 50 and older to state they did not receive a bivalent vaccine because they were not worried about COVID-19 and because they were not required to do so by school or work.
Figure 9: Reasons for not yet having received a bivalent COVID-19 vaccine by race and ethnicity, among adults with 1+ dose of COVID-19 vaccine (Household Pulse Survey, March-April 2023)
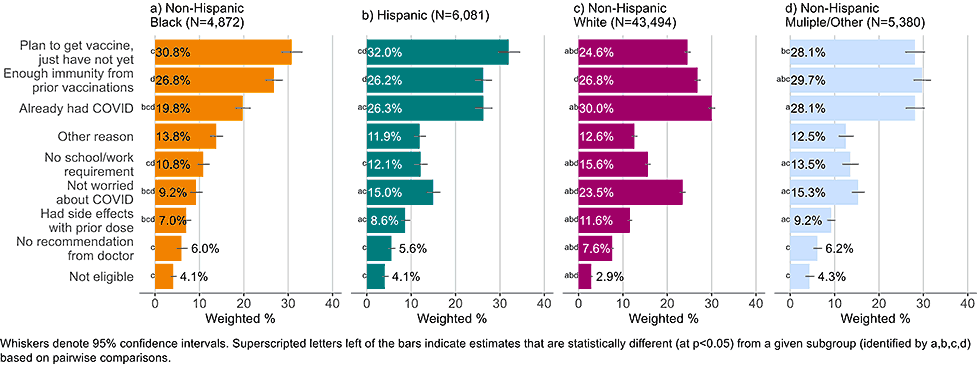
- White non-Hispanic adults were also more likely to list not being worried about COVID-19 as reason for not getting a bivalent vaccine (23.5%) than non-Hispanic, Black adults (9.2%), Hispanic adults (15.0%), and non-Hispanic adults of other or multiple race and ethnicities (15.3%).
Figure 10: Reasons for not yet having received a bivalent COVID-19 vaccine by income, among adults with 1+ dose of COVID-19 vaccine (Household Pulse Survey, March-April 2023)
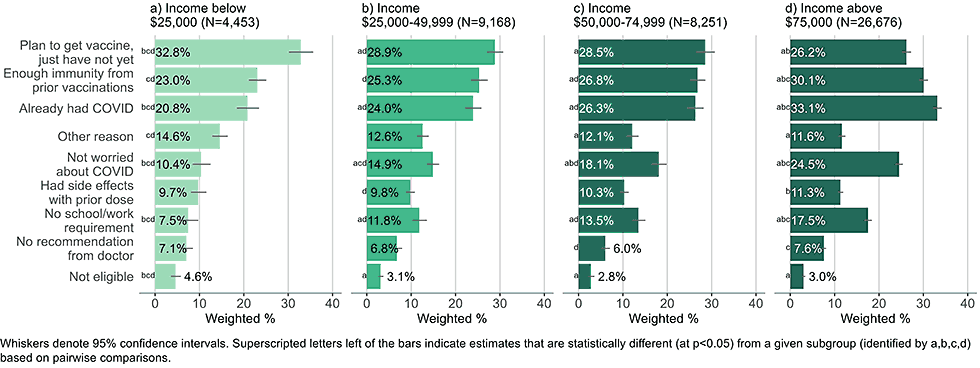
- Higher income adults were more likely to list they had immunity due to prior vaccinations or COVID-19 illness, were not worried about the disease, or had no school/work requirements as reasons for non-vaccination than low-income adults.
Figure 11: Reasons for not yet having received a bivalent COVID-19 vaccine by insurance status, among adults with 1+ dose of COVID-19 vaccine (Household Pulse Survey, March-April 2023)
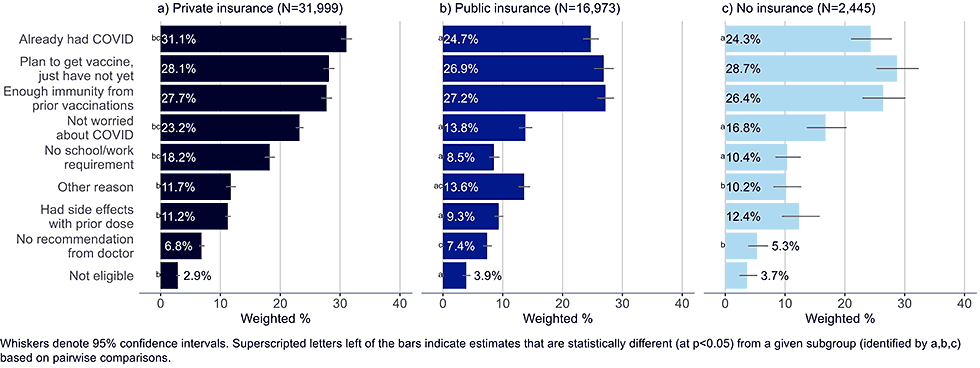
- Adults with private insurance were also more likely than those with public insurance to list having had side effects with a prior dose.
- Adults with public insurance were more likely than those with private insurance to believe they were ineligible for the bivalent vaccine, though less than 4% in any of the insurance groups selected this as a reason for not getting vaccinated.
- Unvaccinated adults largely reflected the same concerns as those with public insurance, but they were less likely to include not having received a recommendation from their doctor as a reason for non-vaccination.
Discussion
- According to our analysis, about a third of all vaccinated adults were open to receiving the bivalent vaccine, but had not yet done so, mainly because they have been too busy or kept forgetting, or because of concerns relating to side effects.
- Forgetting to get the vaccine or planning on getting one and just not having done so yet, were commonly selected reasons by Omnibus and HPS respondents and are in line with findings by a prior study on bivalent booster vaccines5, suggesting adults are open to getting an updated COVID-19 vaccine, but have not made it a priority.
- High rates of openness to the bivalent vaccine were also found among groups with low coverage, such as adults who are young, Hispanic, uninsured, or have a high school degree or less education. These groups were also among the leading subpopulation open to COVID-19 vaccination in general6.
- For groups with low coverage and/or high openness (except for young adults, who mainly were too busy or kept forgetting about getting the vaccine), the most common concern was the possibility of future serious side effects that are as of yet unknown. Other commonly cited concerns were lack of a healthcare provider recommendation, having enough immunity through earlier doses or COVID-19 illness, and being concerned about the vaccine's effectiveness.
- Adults age 65 years and older, who represent one of the groups at highest risk of severe COVID-197, were particularly concerned with not having received a healthcare provider recommendation. Older adults (50 years and above) also commonly reported having received enough COVID-19 doses, suggesting vaccination fatigue among those who have received the highest number of doses.
- Bivalent vaccines have been proven safe and effective, increasing protection against disease8 and reducing serious outcomes of COVID-19 illness9. Making vaccinations easy and convenient and strengthening communication about safety and efficacy, particularly on the safety of mRNA technology and through trusted health care providers, could help reduce some obstacles to achieving higher bivalent vaccination coverage.
Methods
Data for this analysis were collected through the IPSOS Knowledge Panel Fand NORC AmeriSpeak GOmnibus Surveys, which use probability-based panels to survey a nationally representative sample of U.S. adults aged 18 years and older on status, intent, knowledge, attitudes, beliefs, and behaviors related to COVID-19 and flu vaccinations. Respondents who had received one or more doses of COVID-19 vaccine were asked the following question: "An updated COVID-19 booster vaccine became available in September 2022 that is known as a 'bivalent' booster. It can better protect against the most recent Omicron subvariants as well as the original COVID-19 virus. Do you have any of the following concerns about getting an updated COVID-19 booster vaccine?" Respondents were asked to choose all that applied out of 17 answer options, including an open-ended option for "Other," as well as an option for "None of the above."H Data and analyses examining concerns and perceptions of the bivalent vaccine were limited to all respondents who had completed the COVID-19 primary vaccination series. Surveys were fielded twice a month by both Ipsos and NORC, with a sample size of at least 1,000 respondents each. Ipsos samples were drawn from an address-based sampling (ABS) methodology. NORC selects U.S. households randomly using area probability and address-based sampling, with a known, non-zero probability of selection from the NORC National Sample Frame. For this analysis data were pooled from eight survey waves, four each from Ipsos and NORC, with a combined sample size of 8,195 respondents. Limiting the sample to respondents who had completed the primary COVID-19 vaccination seriesIleft a total of 6,271 respondents. Analyses on the subset of adults who are open to receiving a bivalent vaccine included a total of 2,139 respondents. The surveys were fielded within the following time frames in 2023: March 10-12, March 16-20, March 17-25, March 30-April 3, April 7-9, April 13-17, April 21-30, April 27-May 1. Cumulative AAPOR response rates for the included waves ranged from 2.1% to 3.4%. All percentages were weighted along several demographic benchmarks according to the Current Population Survey (CPS)J.
The Census Bureau launched the Household Pulse Survey in April 2020 to create near real-time data on the impact of the COVID-19 pandemic on U.S. households. It is a 20-minute self-response, online survey that samples households out of the Census Bureau's Master Address File, proportional to the population for 66 independent geographic sample areas and adjusted to create independent estimates for states and the fifteen largest metropolitan statistical areasK. Final weights include nonresponse adjustments (among others) and raking to adjust to population estimates by age, sex, race and ethnicityL. The response rate was 6.9% in the first wave and 5.9% in the second wave. For this analysis, data were pooled from two survey waves with a combined sample size of 134,665 respondents. Limiting the sample to respondents who had received at least one COVID-19 vaccination but not yet a bivalent booster vaccine left 61,012 respondentsM. The two waves of HPS included in the analysis were fielded March 1-13, 2023, and March 29-April 10, 2023.
For both surveys, differences among groups noted in this report represent statistically significant differences, determined using t-tests with significance set at p<0.05. All analyses were conducted using R Version 4.1.2
Limitations
The findings in this study are subject to several limitations. All responses are self-reported and may overestimate vaccination coverage and intent due to social desirability bias. Low survey response rates could introduce selection bias. While the sampling procedure and post-stratification weighing mitigate selection effects, some bias may persist. The analysis of some subgroups in the Omnibus data is based on small sample sizes, making results less reliable. All figures include whiskers to indicate 95% confidence intervals around the point estimate.
- https://www.ipsos.com/en-us/solutions/public-affairs/knowledgepanel
- https://amerispeak.norc.org/
- Household Pulse Survey (COVID-19) (census.gov)
- Based on self-reported answers to the following question: “An updated COVID-19 booster vaccine became available in September 2022 that is known as a “bivalent” booster. It can better protect against the most recent Omicron subvariants as well as the original COVID-19 virus. How likely are you to get this updated COVID-19 booster vaccine?” Answer choices included: “Already got an updated booster vaccine,” “Definitely will get an updated booster vaccine,” “Probably will get an updated booster vaccine,” “Not sure if I will get an updated booster vaccine,” “Probably will not get an updated booster vaccine,” “Definitely will not get an updated booster vaccine.”
- Respondents who had received at least one dose of COVID-19 vaccine, but had not received a bivalent booster were asked: “On September 1st, 2022, an updated COVID-19 booster became available. Which of the following, if any, are reasons that you have not received an updated COVID-19 booster dose? Select all that apply.” Response options included “I am not yet eligible to receive an updated COVID-19 booster dose,” “I plan to get a booster and am eligible, but haven’t yet,” “I think I have enough immunity to COVID-19 from prior doses of the vaccine,” “I’m not worried about getting COVID-19,” “My doctor has not recommended it,” “I already had COVID-19,” “I am not required to get a COVID-19 booster (by my work or school),” “I experienced side effects from my previous dose(s) of the COVID-19 vaccine,” “Other (please specify).”
- https://www.ipsos.com/en-us/solutions/public-affairs/knowledgepanel
- https://amerispeak.norc.org/
- Response options included: “I was/am concerned about its effectiveness,” “I was/am worried about mild short-term side effects, such as fever and fatigue,” “I was/am worried about heart-related issues, blood clots, or a stroke,” “I was/am worried about fertility-related issues,” “I was/am worried about unknown serious side effects,” “I had a bad reaction after my previous vaccination,” “I was/am worried about the costs of the vaccine or other related costs (travel, childcare, taking time off),” “I didn’t/don’t know if I was/am eligible,” “I had/have not received a recommendation from my doctor,” “I didn’t/don’t feel that the updated booster had been studied enough (e.g., lack of human trial data),” “I did not/do not trust the government or pharmaceutical companies,” “I do not need the updated booster because I had COVID-19 and have antibodies,” “I have already gotten enough COVID-19 doses,” “I was/am too busy and/or I kept/keep forgetting.”
- Adults who are not immunocompromised and completed a 2-dose primary mRNA COVID-19 vaccine series and adults who are immunocompromised and received a 3-dose mRNA COVID-19 vaccine series are defined as having completed the primary series. For immunocompromised adults whose initial vaccine was a Johnson & Johnson Janssen or Novavax vaccine, primary series completion is defined as receipt of a two-dose series while for those who are not immunocompromised, a single Janssen or Novavax vaccine is defined as sufficient to complete the primary series.
- Current Population Survey (CPS) (census.gov)
- According to the Office of Management and Budget’s CBSA (Core Based Statistical Area) classification.
- More information about HPS methodology can be found at: Household Pulse Survey Technical Documentation (census.gov)
- Vaccination status is self-reported and based on the answer to the following questions: “Have you received at least one dose of a COVID-19 vaccine?” and “How long ago was your most recent dose of the COVID-19 vaccine or booster?,” where respondents who answered their last dose was after September 1,2022, were classified as having received a bivalent booster dose and all other respondents who answered they had received at least one COVID-19 vaccination (but not after September 1, 2022) were classified as vaccinated, but not yet having received a bivalent booster dose. The HPS does not provide enough information to assess whether respondents have completed the COVID-19 primary series.
- CDC recommends the first updated COVID-19 booster. Available at: https://www.cdc.gov/media/releases/2022/s0901-covid-19-booster.html. Accessed February 7, 2023.
- Chalkias S, Harper C, Vrbicky K, et al. A Bivalent Omicron-Containing Booster Vaccine against Covid-19. N Engl J Med. 2022 ;387(14):1279-1291.
- Lu P, Zhou T, Santibanez TA, et al. COVID-19 bivalent booster vaccination coverage and intention to receive booster vaccination among children and adults, United States, October–November 2022. MMWR Morb Mortal Wkly Rep. 2023.
- Updated COVID-19 Vaccines for Use in the United States Beginning in Fall 2023. Available at: https://www.fda.gov/vaccines-blood-biologics/updated-covid-19-vaccines-use-united-states-beginning-fall-2023. Accessed August 11, 2023.
- Sinclair AH, Taylor MK, Weitz JS, Beckett SJ, Samanez-Larkin GR. Reasons for Receiving or Not Receiving Bivalent COVID-19 Booster Vaccinations Among Adults — United States, November 1–December 10, 2022. MMWR Morb Mortal Wkly Rep 2023;72:73–75.
- Omari A, Boone KD, Zhou T, Lu PJ, Kriss JL, Hung MC, Carter RJ, Black C, Weiss D, Masters NB, Lee JT, Brewer NT, Szilagyi PG, Singleton JA. Characteristics of the Moveable Middle: Opportunities Among Adults Open to COVID-19 Vaccination. Am J Prev Med. 2023 May;64(5):734-741.
- Wong MK, Brooks DJ, Ikejezie J, et al. COVID-19 Mortality and Progress Toward Vaccinating Older Adults — World Health Organization, Worldwide, 2020–2022. MMWR Morb Mortal Wkly Rep 2023;72:113–118.
- Tenforde MW, Weber, ZA, Natarajan K, et al. Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19–Associated Emergency Department or Urgent Care Encounters and Hospitalizations Among Immunocompetent Adults – VISION Network, Nine States, September–November 2022. MMWR Morb Mortal Wkly Rep. 2022;71(51-52):1616-24.
- Johnson AG, Linde L, Ali AR, et al. COVID-19 Incidence and Mortality Among Unvaccinated and Vaccinated Persons Aged ≥12 Years by Receipt of Bivalent Booster Doses and Time Since Vaccination — 24 U.S. Jurisdictions, October 3, 2021–December 24, 2022. MMWR Morb Mortal Wkly Rep 2023;72:145–152.
