At a glance
- Among 84,711 pregnant people in this study, 73.7% completed the primary COVID-19 vaccine series before or during pregnancy; booster dose coverage for those who completed a primary series was 58.9% and was also lowest among persons aged 18-24 years and non-Hispanic Black persons.
- Findings indicate a continued need to improve COVID-19 vaccination coverage among pregnant people, especially in younger people and Black people.
Summary
Pregnant and recently pregnant people are at an increased risk for severe illness and pregnancy complications from COVID-19, including increased admission to an intensive care unit, receipt of mechanical ventilation, or death1234. COVID-19 vaccines have been available since December 14, 2020, and recommended for all people who are pregnant, breastfeeding, or trying to get pregnant as of August 11, 2021. Additional doses of the COVID-19 vaccine were recommended beginning on August 13, 2021, for persons who are immunocompromised and for all others in November 202156. Despite these recommendations and expanding evidence supporting the safety and benefits of vaccination for pregnant women and their infants, COVID-19 vaccination coverage among pregnant people from April through November 2021 was 45.1%, substantially lower than among non-pregnant people of reproductive age (64.9%)7. While prior studies have shown COVID-19 vaccination coverage among pregnant people since the beginning of vaccine availability, these studies did not examine additional key characteristics of pregnant people that are needed to provide more targeted vaccine planning and effective communication78. The objectives of this study are to assess COVID-19 vaccination coverage, including monovalent booster dose coverage, among pregnant people by key characteristics, and examine the timing of booster vaccination relative to pregnancy. Electronic health record data from eight Vaccine Safety Datalink health care organizations were used to assess COVID-19 vaccination coverage among people aged 18-49 years pregnant at any time from January 1 through April 30, 2022. Receipt of COVID-19 vaccination by key demographics, high-risk conditions for severe illness from COVID-19 in addition to pregnancy, pregnancy comorbidities, history of COVID-19 disease, and timing of monovalent booster (referred to as booster throughout the report) receipt relative to pregnancy were examined. Among 84,711 pregnant people, 73.7% completed the primary COVID-19 vaccine series before or during pregnancy. Primary series vaccination coverage was lowest among those aged 18-24 years (51.7%) and non-Hispanic Black people (60.1%). Overall booster dose coverage for those who completed a primary series was 58.9% and was also lowest among persons aged 18-24 years and non-Hispanic Black persons and followed similar patterns as the primary series. These findings indicate a continued need to improve COVID-19 vaccination coverage among pregnant people, especially in younger people and Black people. It continues to remain important that pregnant people stay up-to-date with COVID-19 vaccinations and receive all recommended doses.
Methods
The Vaccine Safety Datalink (VSD) is a collaboration between the Centers for Disease Control and Prevention's Immunization Safety Office and nine integrated healthcare systems (sites), in which eight sites from six states provide data to monitor the safety of vaccines and conduct studies about adverse events following vaccination9. Electronic health record (EHR) and claims data capture vaccination records and detailed information on demographics, diagnoses, comorbidities, and healthcare utilization. COVID-19 vaccination data systematically collected by VSD sites include information on vaccines administered both within the healthcare systems and externally. External vaccination information is received through medical or pharmacy claims or data exchange with jurisdictional Immunization Information Systems10.
This analysis used the VSD's validated dynamic pregnancy algorithm (DPA) to identify and estimate gestational age for pregnancies, using the International Classification of Diseases, Tenth Edition (ICD-10-CM) diagnosis codes, procedure codes, estimated dates of delivery, and last menstrual period dates from the EHR and claims data11. Individuals identified by the DPA as pregnant at any time from January 1, 2022, through April 30, 2022, with continuous health plan enrollment from one year prior to the start of pregnancy through the end of data collection, were included in the analysis. Pregnancies that ended in spontaneous or therapeutic abortion, ended prior to 14 weeks gestation, or pregnancies where start date could not be estimated were excluded. COVID-19 vaccination data were collected starting on December 14, 2020. Both pregnancy and vaccination data were extracted through July 16, 2022, to allow for identification of pregnancies starting late in the study period and potential delays in receipt of vaccination data.
Completion of a primary COVID-19 vaccination series was defined as receipt of two doses of Pfizer-BioNTech or Moderna vaccines or one dose of Janssen (Johnson & Johnson) vaccine either before or during a pregnancy. Booster dose coverage (receipt of one additional dose of Pfizer-BioNTech, Moderna, or Janssen vaccine) was assessed among people who completed the primary series and were eligible for a booster dose. We could not differentiate booster doses from an additional primary series dose (as recommended for moderately or severely immunocompromised persons)12; thus, both are included in the booster dose coverage analysis. Data on bivalent booster coverage, authorized on September 1, 2022, are outside of our study timeframe.
COVID-19 vaccination coverage for both primary series and booster doses was estimated among pregnant people aged 18–49 years overall and by age group at pregnancy start, race and ethnicity, high-risk conditions for severe illness due to COVID-19 in addition to pregnancy, pregnancy comorbidities, and history of COVID-19 disease. Booster dose coverage was also estimated by the timing of vaccination relative to pregnancy and pregnancy trimester. High-risk conditions demonstrated to increase the risk for severe illness due to COVID-1913 were ascertained using ICD-10-CM diagnosis codes from EHR or claims data and included qualifying conditions beginning 12 months prior to pregnancy start date through the end of pregnancy or, for ongoing pregnancies, the end of data collection (Supplemental Table). Pregnancy comorbidities included hypertension complicating pregnancy, excessive vomiting, diabetes during pregnancy, alcohol or drug dependence during pregnancy, maternal smoking, or obesity in pregnancy. These were assessed using diagnosis codes during the pregnancy episode of interest. Individuals were classified as having a history of COVID-19 disease if they had an ICD-10-CM diagnosis code for COVID-19 (U07.1), an internal diagnostic code specific to COVID-19 disease, or a positive SARS-CoV-2 laboratory test result from January 20, 2020, through the date of first vaccination, or the end of the study period (April 30, 2022) for unvaccinated individuals.
Supplemental Table. High-risk Conditions Identified to Cause Severe Illness from COVID-19, In Addition to Pregnancy
| Any high-risk for covid condition, excluding pregnancy |
| Chronic lung conditions including COPD, asthma (moderate-severe), cystic fibrosis, pulmonary hypertension, pulmonary fibrosis |
| Cancer |
| Chronic kidney disease |
| Neurologic conditions such as dementia |
| Type 1 or 2 diabetes mellitus |
| Disabilities including down syndrome, cerebral palsy, intellectual disabilities and ADHD. |
| Heart disease and/or hypertension. Includes heart failure, coronary artery disease, cardiomyopathies |
| Immunocompromised state from immunodeficiencies or HIV |
| Liver disease |
| Obesity (body mass index > 30, prior to pregnancy) |
| Sickle cell disease, Thalassemia |
| Immunocompromised state from solid organ, blood or bone marrow transplant |
| Cerebrovascular disease |
| Substance Use |
| Mental Health Conditions |
| Tuberculosis |
Definitions: HR= High-risk
Statistical Analysis
Demographic information and pregnancy characteristics were described using frequencies and percentages for all pregnant people, and separately for those who completed the primary series and received a booster dose. Coverage was calculated as percent with the respective vaccination (primary series or booster) out of the eligible population. All analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC).
Results
Among 84,711 pregnant people, most were aged 25–34 years (60.9%) and identified as either Hispanic or Latino (Hispanic) (34.8%) or Non-Hispanic (NH) White (33.8%). Nearly half of the population had high-risk conditions for severe illness due to COVID-19 (45.7%) or had pregnancy comorbidities (47.0%), and almost one quarter had a history of COVID-19 disease (24.0%) (Table).
Table. Completion of COVID-19 primary series* and COVID-19 monovalent booster vaccination among pregnant people who completed COVID-19 primary series†, by select characteristics — Vaccine Safety Datalink, United States, January–April 2022§
| Characteristics | Total number of pregnant people | Completed primary series | Received booster dose among those who completed primary series† | Received booster dose during pregnancy among those who received booster dose¶ |
| N (col %) | N (row %) | N (row %) | N (row %) | |
| Total | 84,711 | 62,420 (73.7) | 36,790 (58.9) | 23,970 (65.2) |
| Age Group | ||||
|---|---|---|---|---|
| 18-24 years | 10,326 (12.2) | 5,337 (51.7) | 1,635 (30.6) | 1,176 (71.9) |
| 25-34 years | 51,604 (60.9) | 38,235 (74.1) | 22,179 (58.0) | 14,696 (66.3) |
| 35-49 years | 22,781 (26.9) | 18,848 (82.7) | 12,976 (68.9) | 8,098 (62.4) |
| Race and Ethnicity** | ||||
| Hispanic or Latino (Hispanic) | 29,449 (34.8) | 20,893 (70.9) | 9,942 (47.6) | 6,730 (67.7) |
| Non-Hispanic Asian | 13,743 (16.2) | 12,704 (92.4) | 9,372 (73.8) | 5,905 (63.0) |
| Non-Hispanic Black | 5,848 (6.9) | 3,517 (60.1) | 1,393 (39.6) | 951 (68.3) |
| Non-Hispanic Other†† | 3,876 (4.6) | 2,800 (72.2) | 1,652 (59.0) | 1,097 (66.4) |
| Non-Hispanic White | 28,634 (33.8) | 20,171 (70.4) | 13,123 (65.1) | 8,529 (65.0) |
| High-risk conditions for severe illness due to COVID-19§§ | ||||
| No | 45,984 (54.3) | 33,784 (73.5) | 19,903 (58.9) | 12,378 (62.2) |
| Yes | 38,727 (45.7) | 28,636 (73.9) | 16,887 (59.0) | 11,592 (68.6) |
| Pregnancy Comorbidities¶¶ | ||||
| No | 44,893 (53.0) | 33,393 (74.4) | 20,783 (62.2) | 12,879 (62.0) |
| Yes | 39,818 (47.0) | 29,027 (72.9) | 16,007 (55.2) | 11,091 (69.3) |
| History of COVID-19 disease*** | ||||
| No | 64,402 (76.0) | 51,494 (80.0) | 30,928 (60.1) | 19,709 (63.7) |
| Yes | 20,309 (24.0) | 10,926 (54.0) | 5,862 (53.7) | 4,261 (72.7) |
*Completion of primary series was defined as receipt of two doses of the Pfizer-BioNTech or Moderna vaccines or a single dose of the Janssen (Johnson & Johnson) vaccine.
†The count and percentage of pregnant people who received a booster dose includes anyone who is pregnant, completed the primary series, and has received another dose of COVID-19 vaccine. This includes individuals who received a monovalent booster dose and individuals who received additional doses as part of primary series.
§Includes individuals who were pregnant during any of the January 1, 2022–April 30, 2022 study period. Eligible pregnancies that ended within the study period, were still included in the numerator and denominator to ascertain cumulative coverage.
¶The denominator includes pregnant people who received a booster dose.
**Numbers do not add up to the total due to missing race and ethnicity for 3,161 pregnant people who completed the primary series.
††Includes American Indian or Alaska Native, Native Hawaiian or Pacific Islander, and multiple or other races.
§§All patients' records from the outpatient and inpatient settings are screened for high-risk conditions in addition to pregnancy that increase the risk of severe COVID-19 illness using ICD-10-CM codes. Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals
¶¶All patients' records from the outpatient and inpatient settings are screened for pregnancy comorbid conditions assessed from diagnosis codes during pregnancy.
***A combination of ICD-10 COVID-19 codes and internal medical diagnostics codes was used to identify persons with a history of COVID-19 illness. Patient records were also screened for positive laboratory tests for COVID-19 illness before receipt of vaccination. The new ICD-10-CM code came into effect on April 1, https://www.cdc.gov/nchs/data/icd/Announcement-New-ICD-code-for-coronavirus-19-508.pdf
Vaccination Coverage
Among pregnant people included in the study, 73.7% completed a primary series before or during pregnancy. Completion with primary series coverage was highest among people aged 35–49 years (82.7%) and NH Asian (92.4%) and lowest among pregnant people aged 18–24 years (51.7%) and NH Black (60.1%). Primary series coverage was similar among pregnant people with (73.9%) and without (73.5%) additional high-risk conditions for severe illness due to COVID-19 and among those with and without pregnancy comorbidities (72.9% and 74.4% respectively). Pregnant people with history of COVID-19 disease were less likely to be vaccinated (54.0%) when compared with pregnant people who did not have history of COVID-19 disease (80.0%) (Table).
Among 62,420 pregnant people who completed a primary series, 58.9% received a booster dose before or during pregnancy. Booster dose coverage patterns were similar to those for completion of primary series and were highest among pregnant people aged 35–49 years (68.9%) and NH Asian (73.8%), and lowest among pregnant people 18–24 years (30.6%) and NH Black (39.6%). Booster dose coverage was similar among pregnant people with (59.0%) and without (58.9%) additional high-risk conditions for severe illness due to COVID-19. Among pregnant people with pregnancy comorbidities, booster dose coverage was 55.2%, compared with 62.2% among those without. Booster dose coverage was also lower among pregnant people with prior history of COVID-19 disease (53.7%) compared with pregnant people with no reported history of COVID-19 disease (60.1%) (Table).
Timing of Booster Dose Receipt
Among 62,420 pregnant people who completed the primary series, 20.5% received the booster before pregnancy, 13.7% received the booster during the first trimester, 16.8% received the booster dose during the second trimester, and 7.9% received the booster dose during the third trimester. Additionally, 41.1% of pregnant people who completed the primary series did not receive a booster dose by the end of the analytic period. Among pregnant people who received a booster dose, uptake was highest before pregnancy and during the second trimester in all age and race and ethnicity groups (Figure).
Figure. Percent of Pregnant People Aged 18–49 years who received a COVID-19 monovalent booster dose* by timing relative to pregnancy, race and ethnicity, and age group – Vaccine Safety Datalink, United States, January–April 2022†
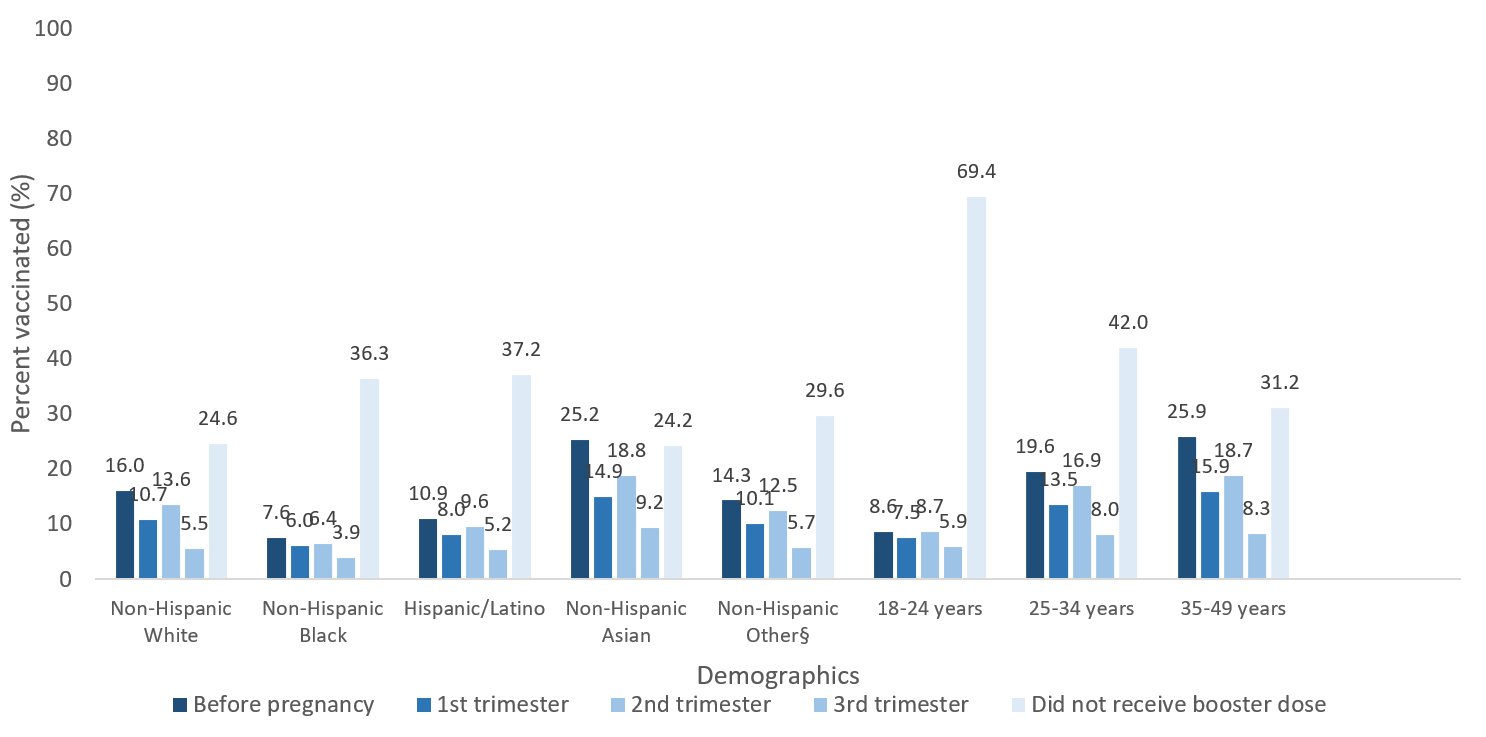
*The count and percentage of pregnant people who received a monovalent booster dose includes anyone who completed the primary vaccine series and has received another dose of COVID-19 vaccine during pregnancy, as of July 16, 2022. This includes individuals who received booster doses and individuals who received additional doses as part of primary series. The time interval between primary series and booster dose was not considered.
†Denominator includes cumulative number of individuals who were pregnant at any time during the period January 1, 2022, through April 30, 2022, and completed the primary vaccine series.
§Includes American Indian or Alaska Native, Native Hawaiian or Pacific Islander, and multiple or other races.
Discussion
Data from the VSD as of July 16, 2022, estimated that the majority of the pregnant people included in the analysis completed the primary series of COVID-19 vaccines, with differences observed by age, race and ethnicity, and history of COVID-19 disease. Among pregnant people, COVID-19 vaccination coverage, including booster dose receipt, has increased since the beginning of vaccine availability7814, but some pregnant people continue to remain unvaccinated and unprotected, indicating possibly persistent vaccine hesitancy. Vaccine hesitancy, not limited to COVID-19 vaccination, is common among pregnant people [15–16]. Proportion of pregnant people vaccinated against COVID-19 is comparable to pregnant people vaccinated against tetanus-toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) (61.2%) and influenza (56.6%)1516. Surveys on COVID-19 vaccination intent show continued vaccine hesitancy among pregnant people, with most currently unvaccinated individuals having no plans to get vaccinated during pregnancy1718. Common reasons reported by pregnant people for not receiving recommended vaccinations include concerns about safety risks to themselves or their infants, side effects, and effectiveness of the vaccines18. Lower COVID-19 vaccination coverage may substantially impact pregnancy outcomes, including increased risk for stillbirth compared with individuals without COVID-19 vaccination19. Therefore, providing additional information on vaccine safety, including safety of vaccination during pregnancy, the impact on vaccination on pregnancy and infant outcomes, and tailored messaging regarding personalized preconceptions and attitudes towards vaccination, may increase uptake among pregnant people.
COVID-19 primary series and booster dose coverage remains lowest among Black and Hispanic pregnant people. However, more recent data indicate that primary series coverage among Hispanic pregnant people is now similar to coverage among NH White pregnant people, but disparities among NH Black pregnant people remain20. Additionally, NH Black and Hispanic pregnant people have lower bivalent booster coverage when compared with other race and ethnicity groups20. Our findings indicate that only three in five Black pregnant people completed a primary series, and approximately two in five Black pregnant people received a booster among those eligible. Similar disparities in vaccination coverage by race and ethnicity have been observed for other maternal vaccinations such as influenza and Tdap vaccines, with lower vaccination coverage among Black and Hispanic people1518. Previous studies indicate several factors, including knowledge, attitudes, and beliefs about vaccines, persistent structural inequities, such as need to delay medical care that disproportionately affect racial and ethnic minority groups, and barriers related to accessing healthcare services, contribute to lower vaccination rates among these populations1821. Lower rates of vaccination among disproportionately impacted populations may also be due to mistrust of government due to historical and ongoing substandard care, lack of flexible work schedules, or limited transportation options22.
Continuing to monitor vaccination coverage, in combination with consistent provider recommendations for vaccination along with recommendations provided by trusted community members, among populations disproportionately affected by COVID-19 disease could decrease disparities in vaccination coverage2324, which is especially important in racial and ethnic groups where higher rates of hospitalizations and death due to COVID-19 disease are observed24.
Overall, more pregnant people were likely to receive the COVID-19 booster dose during pregnancy, as opposed to before pregnancy. Demographic subgroups with the lowest primary series coverage before and during pregnancy were also the least likely to receive a booster dose (e.g., NH Black, Hispanic, and pregnant people aged 18–24 years). Uptake was lower during the first trimester compared to the second trimester, possibly indicating that women are leerier of being vaccinated early in pregnancy. Our study also indicated that uptake decreased during the third trimester for all age groups and all race and ethnicity groups, which is consistent with other studies18 and likely related to the timing of the booster dose recommendations and the pregnancy window in our cohort. However, vaccination against COVID-19 is recommended during pregnancy regardless of gestational age56. Data also show that COVID-19 vaccination during pregnancy can lead to the transfer of antibodies through the placenta and breast milk, which may confer immunity to newborns and is associated with a reduced risk of hospitalization among infants younger than 6 months of age2526. However, waning immunity from primary series and booster doses has been observed27, and it remains important that pregnant people stay up-to-date on all recommended COVID-19 vaccinations, including getting a bivalent booster when indicated.
Limitations
This study is subject to several limitations. First, the findings may not be generalizable to all pregnant persons in the United States. Second, vaccination status may be misclassified in the VSD if pregnant persons receive vaccinations outside of the participating delivery systems and state registry catchment area. Third, this analysis did not differentiate between an additional primary series dose and a booster dose. Fourth, the DPA may result in some misclassification of pregnancy outcomes and dates11. Fifth, not all positive COVID-19 cases may have been identified by the VSD since at home tests may remain unreported to healthcare providers, which may result in misclassification of history of COVID-19 disease. Lastly, this study does not assess the more recently recommended bivalent booster vaccine.
These findings provide insight into COVID-19 vaccination coverage among pregnant people by many factors, including race and ethnicity, pregnancy comorbidities, having high-risk conditions for severe illness due to COVID-19 in addition to pregnancy, history of COVID-19 disease and timing of booster doses relative to pregnancy. COVID-19 vaccinations lower the risk of serious illness due to COVID-19 in pregnant people and messaging about the importance of vaccination that is strong, concise, consistent, and delivered by a trusted source is essential. Continued focus to improve vaccination in this population overall, as well as specific sub-populations with lower vaccination coverage such Black and Hispanic people is needed. Encouraging healthcare providers to have conversations about the importance of vaccination prior to and during any trimester of pregnancy, highlighting the safety and effectiveness of the COVID-19 vaccines, and recommending the vaccine to all pregnant people may help increase acceptance and uptake of the vaccine. Additionally, addressing barriers to access and vaccine hesitancy could increase confidence and acceptance of COVID-19 vaccines among pregnant people.
Authors
Mehreen Meghani, MPH,; Hilda Razzaghi, PhD; Bradley Crane, MS; Eric S. Weintraub, MPH; Allison L. Naleway, PhD; Stephanie A. Irving, MHS; Tia L. Kauffman, MPH; Matthew F. Daley, MD; Malini DeSilva, MD, MPH; Darios Getahun, MD, PhD; Sungching C. Glenn, MS; Simon J. Hambidge, MD, PhD; Kayla E. Hanson, MPH; Tat'Yana Kenigsberg, MPH; Heather S. Lipkind, MD, MS; Jennifer Nelson, PhD; Gabriela Vazquez-Benitez, PhD; Ousseny Zerbo, PhD; Carla L. Black, PhD
Centers for Disease Control and Prevention; Kaiser Permanente Center for Health Research, Portland, Oregon; Kaiser Permanente Colorado, Denver, CO; Health Partners Institute, Minneapolis, MN; Kaiser Permanente Southern California, Pasadena, CA; Denver Health, Denver, CO; Marshfield Clinic Research Institute, Marshfield, WI; Yale University, New Haven, CT; Kaiser Permanente Washington, Seattle, WA; Vaccine Study Center, Kaiser Permanente Northern California, Oakland, CA
Disclaimer
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi:10.1136/bmj.m3320
- Centers for Disease Control and Prevention. People with certain medical conditions. Accessed February 10, 2022. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html
- Kasehagen L, Byers P, Taylor K, et al. COVID-19-Associated Deaths After SARS-CoV-2 Infection During Pregnancy – Mississippi, March 1, 2020-October 6, 2021. MMWR Morb Mortal Wkly Rep 2021;70(47):1646-1648. doi:10.15585/mmwr.mm7047e2
- Prasad, S., Kalafat, E., Blakeway, H. et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat Commun 13, 2414 (2022). https://doi.org/10.1038/s41467-022-30052-w
- Centers for Disease Control and Prevention. COVID-19 vaccines while pregnant or breastfeeding. Accessed June 8, 2022. COVID-19 Vaccines While Pregnant or Breastfeeding (cdc.gov)
- The American College of Obstetricians and Gynecologists. ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals. Available at: https://www.acog.org/news/news-releases/2021/07/acogsmfm-recommend-covid-19-vaccination-for-pregnant-individuals. September 27, 2021.
- Razzaghi, H., et al., COVID-19 vaccination coverage and intent among women aged 18-49 years by pregnancy status, United States, April-November 2021. Vaccine, 2022. 40(32): p. 4554-4563. DOI: 10.1016/j.vaccine.2022.06.029.
- Razzaghi H, Meghani M, Pingali C, et al. COVID-19 Vaccination Coverage Among Pregnant Women During Pregnancy — Eight Integrated Health Care Organizations, United States, December 14, 2020–May 8, 2021. MMWR Morb Mortal Wkly Rep 2021;70:895–899. DOI: http://dx.doi.org/10.15585/mmwr.mm7024e2
- Baggs, J. Gee, E. Lewis, G. Fowler, P. Benson, T. Lieu, et al. The vaccine safety datalink: a model for monitoring immunization safety. 2011 May;127 Suppl 1:S45-53. doi: 10.1542/peds.2010-1722H. Epub 2011 Apr 18. PMID: 21502240.
- Groom HC, Crane B, Naleway AL, Weintraub E, Daley MF, et al. Monitoring vaccine safety using the Vaccine Safety Datalink: Assessing capacity to integrate data from Immunization Information Systems. Vaccine. 2022 Jan 31;40(5):752-756
- Naleway AL, Crane B, Irving SA, et al. Vaccine Safety Datalink infrastructure enhancements for evaluating the safety of maternal vaccination. Therapeutic Advances in Drug Safety. January 2021. doi:1177/20420986211021233
- Centers for Disease Control and Prevention. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. Accessed March 1, 2023. Clinical Guidance for COVID-19 Vaccination | CDC
- Centers for Disease Control and Prevention. Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals. Accessed October 28, 2022. Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals | CDC
- Razzaghi H, Meghani M, Crane B, et al. Receipt of COVID-19 Booster Dose Among Fully Vaccinated Pregnant Individuals Aged 18 to 49 Years by Key Demographics. JAMA. 2022;327(23):2351–2354. doi:10.1001/jama.2022.6834
- Razzaghi H, Kahn KE, Black CL, et al. Influenza and Tdap Vaccination Coverage Among Pregnant Women — United States, April 2020. MMWR Morb Mortal Wkly Rep 2020;69:1391–1397. DOI: http://dx.doi.org/10.15585/mmwr.mm6939a2
- Chamberlain AT, Seib K, Ault KA, et al. Factors Associated with Intention to Receive Influenza and Tetanus, Diphtheria, and Acellular Pertussis (Tdap) Vaccines during Pregnancy: A Focus on Vaccine Hesitancy and Perceptions of Disease Severity and Vaccine Safety. PLoS Curr 2015;7.
- Huddleston HG, Jaswa EG, , et al. COVID-19 vaccination patterns and attitudes among American pregnant individuals. Am J Obstet Gynecol MFM 2022;4:100507.
- Centers for Disease Control and Prevention. Flu and Tdap Vaccination Coverage Among Pregnant Women – United States, April 2022. Flu, Tdap, and COVID-19 Vaccination Coverage Among Pregnant Women – United States, April 2022 | FluVaxView | Seasonal Influenza (Flu) | CDC
- DeSisto CL, Wallace B, Simeone RM, et al. Risk of Stillbirth Among Women With and Without COVID-19 Delivery Hospitalization — United States, March 2020–September 2021. MMWR Morb Mortal Wkly Rep 2021;70:1640–1645. DOI: http://dx.doi.org/10.15585/mmwr.mm7047e1
- Centers for Disease Control and Prevention. COVID-19 Vaccination Among Pregnant People aged 18-49 years overall, by race and ethnicity, and date reported to CDC – Vaccine Safety Datalink , United States. Accessed on March 1, 2023. CDC COVID Data Tracker: Vaccination Among Pregnant People
- Brewer, LI, Ommerborn, MJ, Nguyen, AL, et al. Structural inequities in seasonal influenza vaccination rates. BMC Public Health. 2021;21: https://doi.org/10.1186/s12889-021-11179-9
- Centers for Disease Control and Prevention. A Guide for Community Partners. Increasing COVID-19 Vaccination Uptake Among Members of Racial and Ethnic Minority Communities. Accessed on April 5, 2023. CDC A Guide for Community Partners-Increasing COVID-19 Vaccine Uptake Among Racial and Ethnic Minority Communities
- Jarrett, C, Wilson, R, O'Leary, M, et al., Strategies for addressing vaccine hesitancy – A systematic review. Vaccine, 2015. 33(34): p. 4180-90.
- Romano SD, Blackstock AJ, Taylor EV, et al. Trends in Racial and Ethnic Disparities in COVID-19 Hospitalizations, by Region — United States, March–December 2020. MMWR Morb Mortal Wkly Rep 2021;70:560–565. DOI: http://dx.doi.org/10.15585/mmwr.mm7015e2
- Halasa NB, Olson SM, Staat MA, et al. Maternal vaccination and risk of hospitalization for COVID-19 among infants. N Engl J Med 2022; 387:109-119. DOI: 10.1056/NEJMoa2204399
- Perl SH, Uzan-Yulzari A, Klainer H, et al. SARS-CoV-2–Specific Antibodies in Breast Milk After COVID-19 Vaccination of Breastfeeding Women. JAMA. 2021;325(19):2013–2014. DOI: https://doi.org/10.1001/jama.2021.5782
- Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19—Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance — VISION Network, 10 States, August 2021—January 2022. MMWR Morb Mortal Wkly Rep 2022;71:255-263. DOI: http://dx.doi.org/10.15585/mmwr.mm7107e2
