At a glance
Supplementary vaccination coverage data from the NIS-Child are included below. The figures show trends of estimated vaccination coverage and estimated percentages of children with no vaccinations. The table shows vaccination coverage comparison estimates for two survey years.
Supplementary Figure 1
Estimated Vaccination Coverage by Age 24 Months,* by Birth Year,† National Immunization Survey-Child, United States, 2012-2020
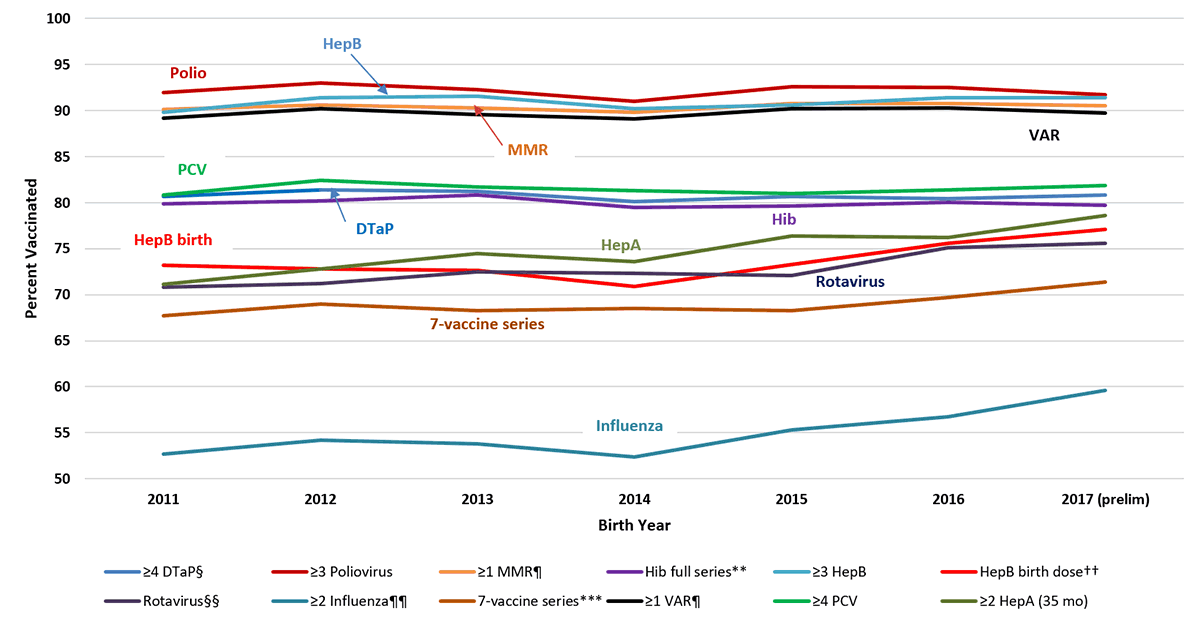
Abbreviations: DTaP = diphtheria, tetanus toxoids, and acellular pertussis vaccine; HepA = hepatitis A vaccine; HepB = hepatitis B vaccine; Hib = Haemophilus influenzae type b conjugate vaccine; MMR = measles, mumps, and rubella vaccine; PCV = pneumococcal conjugate vaccine; VAR = varicella vaccine.
*Includes vaccinations received by age 24 months (before the day the child turns 24 months), except for the HepB birth dose, rotavirus vaccination, and ≥2 HepA doses by 35 months. For all vaccines except the HepB birth dose and rotavirus vaccination, the Kaplan-Meier method was used to estimate vaccination coverage to account for children whose vaccination history was ascertained before age 24 months (35 months for ≥2 HepA doses).
† Children born in 2011 are included in survey years 2012, 2013, and 2014; children born in 2012 are included in survey years 2013, 2014, and 2015; children born in 2013 are included in survey years 2014, 2015, and 2016, children born in 2014 are included in survey years 2015, 2016, and 2017; children born in 2015 are included in survey years 2016, 2017, and 2018; children born in 2016 are included in survey years 2017, 2018, and 2019; children born in 2017 are included in survey years 2018, 2019 and 2020; children born in 2018 are included in survey years 2019 and 2020 (data from survey year 2021 are not yet available).
§ Includes children who may have been vaccinated with diphtheria and tetanus toxoids vaccine or diphtheria, tetanus toxoids, and pertussis vaccine.
¶ Includes children who may have been vaccinated with measles, mumps, rubella, and varicella combination vaccine.
** Hib full series: primary series and booster dose, which includes receipt of ≥3 or ≥4 doses, depending on product type received.
†† One dose HepB administered from birth through age 3 days.
§§ Includes ≥2 doses of Rotarix monovalent rotavirus vaccine (RV1), or ≥3 doses of RotaTeq pentavalent rotavirus vaccine (RV5). The maximum age for the final rotavirus dose is 8 months, 0 days.
¶¶ Doses must be at least 24 days apart (four weeks with a four-day grace period); doses could have been received during two influenza seasons.
*** The combined 7-vaccine series (4:3:1:3*:3:1:4) includes ≥4 doses of DTaP, ≥3 doses of poliovirus vaccine, ≥1 dose of measles-containing vaccine, the full series of Hib (≥3 or ≥4 doses, depending on product type), ≥3 doses of HepB, ≥1 dose of VAR, and ≥4 doses of PCV.
Supplementary Figure 2
Estimated percentage of children who received no vaccinations by age 24 months,* by birth year† – National Immunization Survey-Child, United States, 2012-2020
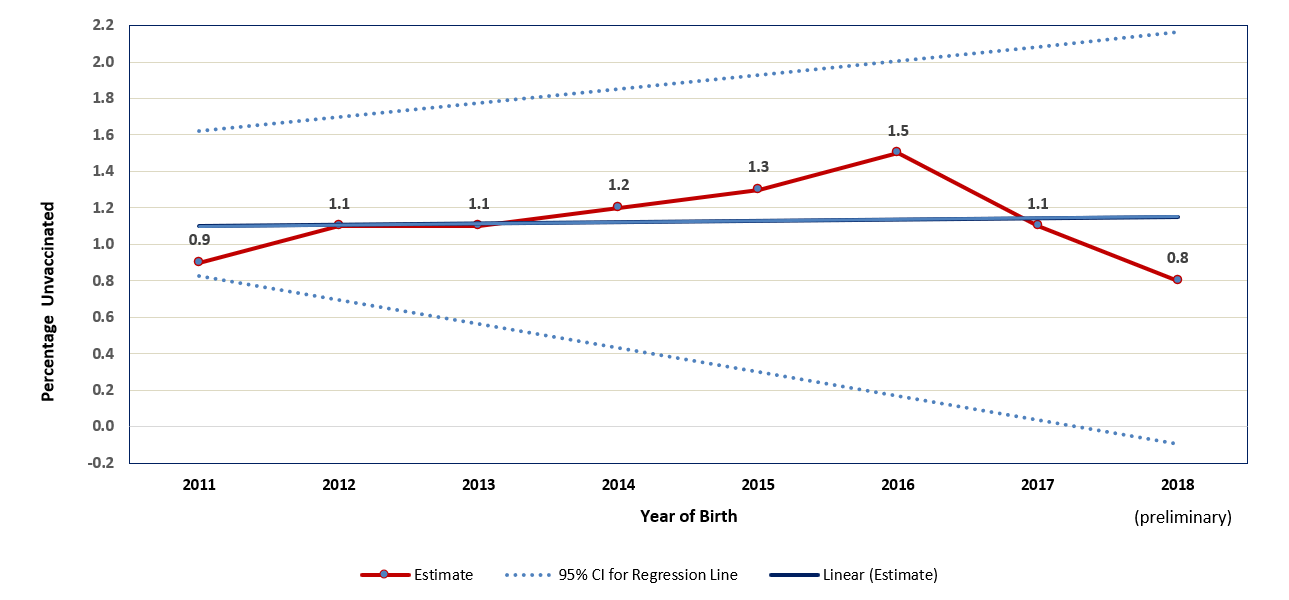
Abbreviations: CI = confidence interval
* Kaplan-Meier techniques are used to calculate estimates by age 24 months.
† Children born in 2011 are included in survey years 2012, 2013, and 2014; children born in 2012 are included in survey years 2013, 2014, and 2015; children born in 2013 are included in survey years 2014, 2015, and 2016, children born in 2014 are included in survey years 2015, 2016, and 2017; children born in 2015 are included in survey years 2016, 2017, and 2018; children born in 2016 are included in survey years 2017, 2018, and 2019; children born in 2017 are included in survey years 2018, 2019, and 2020; children born in 2018 are included in survey years 2019 and 2020 (data from survey year 2021 are not yet available). Estimated linear relationship between year of birth and vaccination status, based on weighted linear regression analysis using the inverse of the estimated variance of each point estimate to construct the weights. Observed percent unvaccinated (95% confidence interval): 2011 0.9 (0.6-1.3); 2012 1.1 (0.8-1.4); 2013 1.1 (0.8-1.4), 2014 1.2 (1.0-1.5); 2015 1.3 (1.1-1.6); 2016 1.5 (1.2-1.8); 2017 1.1 (0.9-1.3) 2018 (preliminary) 0.8 (0.6-1.1). Estimated percentage point change per year: 0.04 (-0.08-0.15).
Supplementary Table
Vaccination coverage estimates for bridging cohort,* National Immunization Survey-Child, United States, 2019-2020
| Survey Year 2019 | Survey Year 2020 | Difference (2020-2019) |
P-value for Test of No Difference | ||||
|---|---|---|---|---|---|---|---|
| Vaccine / Doses | Estimate | SE | Estimate | SE | Estimate | SE | |
| ≥3 DTaP by 19 months | 91.4 | 0.70 | 93.1 | 0.48 | 1.7 | 0.85 | 0.042 |
| ≥4 DTaP by 19 months | 71.1 | 1.10 | 72.8 | 0.83 | 1.8 | 1.38 | 0.200 |
| ≥3 Poliovirus by 19 months | 90.1 | 0.74 | 91.8 | 0.53 | 1.7 | 0.91 | 0.067 |
| ≥1 MMR by 19 months | 87.3 | 0.88 | 88.6 | 0.63 | 1.3 | 1.08 | 0.236 |
| Hib primary series† by 19 months | 90.5 | 0.75 | 92.7 | 0.49 | 2.2 | 0.90 | 0.016 |
| Hib full series† by 19 months | 74.7 | 1.07 | 75.2 | 0.83 | 0.5 | 1.35 | 0.707 |
| HepB birth dose§ | 77.5 | 1.01 | 77.7 | 0.79 | 0.2 | 1.28 | 0.869 |
| ≥3 HepB by 19 months | 89.9 | 0.77 | 91.0 | 0.53 | 1.1 | 0.94 | 0.224 |
| ≥1 VAR by 19 months | 86.5 | 0.89 | 87.9 | 0.63 | 1.4 | 1.09 | 0.209 |
| ≥3 PCV by 19 months | 90.0 | 0.76 | 92.0 | 0.50 | 2.0 | 0.91 | 0.028 |
| ≥4 PCV by 19 months | 77.7 | 1.03 | 78.7 | 0.78 | 1.0 | 1.29 | 0.432 |
| ≥1 HepA by 19 months | 82.4 | 0.91 | 82.1 | 0.72 | -0.3 | 1.16 | 0.776 |
| ≥2 HepA by 19 months | 28.1 | 1.10 | 27.2 | 0.78 | -0.9 | 1.35 | 0.500 |
| Rotavirus¶ by 19 months | 76.6 | 0.98 | 76.5 | 0.80 | -0.2 | 1.27 | 0.904 |
| ≥2 Influenza** by 19 months | 56.6 | 1.15 | 55.1 | 0.91 | -1.6 | 1.47 | 0.290 |
| Combined 7-vaccine series†† by 19 months | 62.6 | 1.18 | 62.4 | 0.90 | -0.2 | 1.48 | 0.890 |
| No vaccinations | 0.7 | 0.10 | 0.8 | 0.12 | 0.1 | 0.16 | 0.514 |
Abbreviations: DTaP = diphtheria, tetanus toxoids, and acellular pertussis vaccine; HepA = hepatitis A vaccine, HepB = hepatitis B vaccine; Hib = Haemophilus influenzae type b conjugate vaccine; MMR = measles, mumps, and rubella vaccine; PCV = pneumococcal conjugate vaccine; SE = standard error; VAR = varicella vaccine
*The bridging birth cohort includes children born from January 2017 to May 2018 who were included in the 2019 National Immunization Survey-Child and children born from January 2017 to May 2018 who were included in the 2020 National Immunization Survey-Child.
† Hib primary series: receipt of ≥2 or ≥3 doses, depending on product type received; full series: primary series and booster dose, which includes receipt of ≥3 or ≥4 doses, depending on product type received.
§ One dose HepB administered from birth through age 3 days.
¶ Includes ≥2 doses of Rotarix monovalent rotavirus vaccine (RV1) or ≥3 doses of RotaTeq pentavalent rotavirus vaccine (RV5). (If any dose in the series is either RotaTeq or unknown, default to 3-dose series.) The maximum age for the final rotavirus dose is 8 months, 0 days.
** Doses must be at least 24 days apart (four weeks with a four-day grace period); doses could have been received during two influenza seasons.
†† The combined 7-vaccine series (4:3:1:3*:3:1:4) includes ≥4 doses of DTaP, ≥3 doses of poliovirus vaccine, ≥1 doses of measles-containing vaccine, the full series of Hib (≥3 or ≥4 doses, depending on product type), ≥3 doses of HepB, ≥1 dose of VAR, and ≥4 doses of PCV.
