Key points
- Coverage for the age-appropriate composite adult vaccination measures for 2019 was low (<45%) for both versions of the composite measure among adults aged ≥19 years and among all race/ethnicity groups for adults aged ≥19 (<50%).
- Coverage for all vaccines differed by race/ethnicity with generally lower coverage among non-White adults compared with White adults.
Summary
The Centers for Disease Control and Prevention (CDC) recommends vaccinations for adults based on age, health conditions, prior vaccinations, and other considerations to prevent morbidity and mortality from vaccine-preventable diseases. Updated vaccination recommendations for adults from CDC are published annually, and Healthy People 2030 (HP2030) objectives include increasing the proportion of adults aged 19 years or older who receive recommended age-appropriate vaccines. Still, vaccination coverage among U.S. adults remains low for most vaccines.
To assess vaccination coverage among adults aged ≥19 years, CDC analyzed data from the National Health Interview Survey (NHIS). NHIS is a continuous, cross-sectional national household survey of the noninstitutionalized U.S. civilian population. In a probability sample of households, interviews are conducted over the course of the year and data are compiled and released on an annual basis. For this report, adult receipt of influenza, pneumococcal, herpes zoster, and Td/Tdap vaccines was assessed using data from 2019 and 2020. A composite adult vaccination quality measure1, which tracks routinely recommended age-appropriate vaccination among adults, was assessed using 2019 data, and trends in adult vaccination were examined during 2010–2020 with a particular focus on vaccination coverage since 2016 to represent recent trends in adult vaccination.
Coverage for the age-appropriate composite adult vaccination measures for 2019 was low (<45%) for both versions of the composite measure among adults aged ≥19 years and among all race/ethnicity groups for adults aged ≥19 (<50%). Coverage for all vaccines differed by race/ethnicity with generally lower coverage among non-White adults compared with White adults. Linear trend tests since 2016 indicated that coverage increased for influenza, herpes zoster and Tdap vaccination and remained stable for pneumococcal and vaccination with any tetanus-containing vaccine.
Substantial improvement in adult vaccination uptake is needed to reduce the burden of vaccine-preventable diseases. Increasing the proportion of adults who receive recommended age-appropriate vaccines and assuring equitable access to and uptake of recommended vaccines is a high-priority public health issue.
Methods
The NHIS is a continuous, cross-sectional national household survey of the noninstitutionalized U.S. civilian population conducted by the U.S. Census Bureau for CDC’s National Center for Health Statistics. Due to data collection difficulties posed by the COVID-19 pandemic, the 2020 NHIS shifted from in-person interviewing to all-telephone interviewing starting in late March and continuing through June 2020. From July through December 2020, data collection in select areas was opened for in-person interviewing, however, NHIS data collection remained predominantly by telephone during this period2. NHIS’s objective is to monitor the health of the U.S. population and provide estimates of health indicators, health care use and access, and health-related behaviors3. Non-institutionalized adults aged ≥19 years with interviews conducted during August 2019–June 2020 (for influenza vaccination), January–December 2020 (for pneumococcal and herpes zoster vaccination), and January–December 2019 (for Td and Tdap vaccination) were included in this analysis. (Information on Td or Tdap vaccination was not collected in the 2020 NHIS.) The total sample of persons aged ≥19 years was 31,633 in 2019 and 31,360 in 2020. The final sample adult core response rate was 59.1% for the 2019 NHIS and 48.9% for the 2020 NHIS. Questions about receipt of vaccinations recommended for adults are asked of one randomly selected adult within each family in the household. Weighted data were used to produce national vaccination coverage estimates. For non-influenza adult vaccination coverage estimates, the weighted proportion of respondents who reported receiving selected vaccinations was calculated. To better assess influenza vaccination coverage each season, the Kaplan-Meier survival analysis procedure was used. Race/ethnicity was categorized as follows: White, Black, Hispanic, Asian, and Other. In this report, persons categorized as White, Black, Asian, or Other race identified as non-Hispanic. Persons categorized as Hispanic might be of any race. Persons characterized as Other include those who identified as American Indian/Alaska Native and persons who identified multiple races. The five race/ethnicity categories are mutually exclusive.
For the adult vaccination composite measure, data from the 2019 NHIS were analyzed to determine estimates for a composite measure of vaccination coverage for select vaccines routinely recommended for all adults aged ≥19 years (Td, Tdap, and influenza vaccine) or indicated based on age (herpes zoster and pneumococcal vaccines), and for three age groups (19–59 years, 60–64 years, and ≥65 years) based on the vaccines recommended for those age groups. Estimates for composite measures were calculated to include any tetanus-toxoid containing vaccine in the past 10 years, with and without influenza vaccination in the past 12 months. Point estimates and 95% confidence intervals (CIs) were calculated by using SUDAAN software (Research Triangle Institute, Research Triangle Park, NC, version 11.0.1) to account for the complex sample design. T-tests were used for comparisons between data years and for comparisons of each level of each respondent characteristic to a chosen referent level (e.g., for race/ethnicity, White was the reference group). Statistical significance was defined as p<0.05. Coverage estimates are not reported for small sample size (n<30) or large relative standard errors (standard error/estimate >0.3).
Results
- Pneumococcal vaccination coverage overall (≥1 dose of PPSV23 or PCV13) among adults aged 19–64 years at increased risk for pneumococcal disease was 23.9% in 2020, similar to the estimate for 2019.
- Coverage among White adults aged 19–64 years at increased risk was higher (26.3%) compared with Hispanic (16.7%) and Asian (13.8%) adults.
- Coverage among White adults aged 19–64 years at increased risk was higher (26.3%) compared with Hispanic (16.7%) and Asian (13.8%) adults.
- Coverage among adults aged ≥65 years was 67.5%, similar to the estimate for 2019.
- Coverage among White adults aged ≥65 years (72.4%) was higher compared with Black (50.8%), Hispanic (48.1%), and Asian (54.9%) adults.
- Coverage among White adults aged ≥65 years (72.4%) was higher compared with Black (50.8%), Hispanic (48.1%), and Asian (54.9%) adults.
- Overall, herpes zoster vaccination coverage among adults aged ≥50 and ≥60 years in 2020 was 29.4% and 39.1%, respectively, higher than estimates for 2019.
- White adults aged ≥50 and ≥60 years had higher coverage compared with Black, Hispanic, and Other adults.
- White adults aged ≥50 and ≥60 years had higher coverage compared with Black, Hispanic, and Other adults.
- Zoster vaccine live (ZVL) coverage in 2020 was 17.8% among adults aged ≥50 years, 4.0% among adults aged 50–59 years, and 25.5% among adults aged ≥60 years, all lower than estimates for 2019.
- Recombinant zoster vaccine (RZV) coverage (≥1 dose) was 14.1% among adults aged ≥50 years, 7.3% among adults aged 50–59 years, and 17.9% among adults aged ≥60 years, all higher than estimates for 2019.
- RZV coverage (at least 2 doses) was 10.8% among adults aged ≥50 years, 13.9% among adults aged ≥60 years, and 15.1% among adults aged ≥65 years, all higher than estimates for 2019.
- RZV coverage (at least 2 doses) was 10.8% among adults aged ≥50 years, 13.9% among adults aged ≥60 years, and 15.1% among adults aged ≥65 years, all higher than estimates for 2019.
- In 2019, the proportion of adults aged ≥19 years reporting having received any tetanus toxoid–containing vaccination during the past 10 years was 62.9%, similar to 2018.
- White adults had higher coverage compared with Black, Hispanic, and Asian adults.
- White adults had higher coverage compared with Black, Hispanic, and Asian adults.
- Among adults aged ≥19 years for whom Tdap vaccination could be assessed specifically, overall coverage was 30.1%, similar to the estimate for 2018.
- In 2019, few adults aged ≥19 years had received all age-appropriate vaccines (including influenza vaccination) included in the composite measure (21.8%).
- Coverage for the composite adult vaccination quality measure was low in all age groups, ranging from 8.2% among adults aged 50–64 years to 27.6% among adults aged 19–49 years.
- Low coverage with herpes zoster vaccine was the primary driver of low coverage among adults aged 50–64 years.
- Low coverage with herpes zoster vaccine was the primary driver of low coverage among adults aged 50–64 years.
- Coverage for the composite adult vaccination quality measure (including influenza vaccination) was lower among Black (15.9%) and Hispanic (17.3%) adults compared with White (23.7%), Asian (23.5%) and Other (25.7%) adults aged ≥19 years.
- Trends in coverage with select vaccines recommended for adults from 2010–2020 are shown in the Figure.
- While coverage for all vaccines except any tetanus-containing vaccine (Td or Tdap) increased since 2010, coverage for several vaccines has plateaued in recent years.
- From 2016–2020, increases in coverage were observed for influenza vaccination among adults aged ≥19 years (annual average percentage point increase: 1.6%, 95% CI 1.2, 2.0); herpes zoster vaccination among adults aged ≥60 years (annual average percentage point increase: 1.2%, 95% CI 0.8, 1.6); and Tdap vaccination among adults aged ≥19 years (annual average percentage point increase: 1.0%, 95% CI 0.4, 1.5). Coverage for pneumococcal vaccination and for any tetanus-containing vaccine (Td or Tdap) remained stable from 2016–2020.
FIGURE. Estimated proportion of adults aged ≥19 years who received selected vaccines, by age group and risk status — National Health Interview Survey, United States, 2010–2020
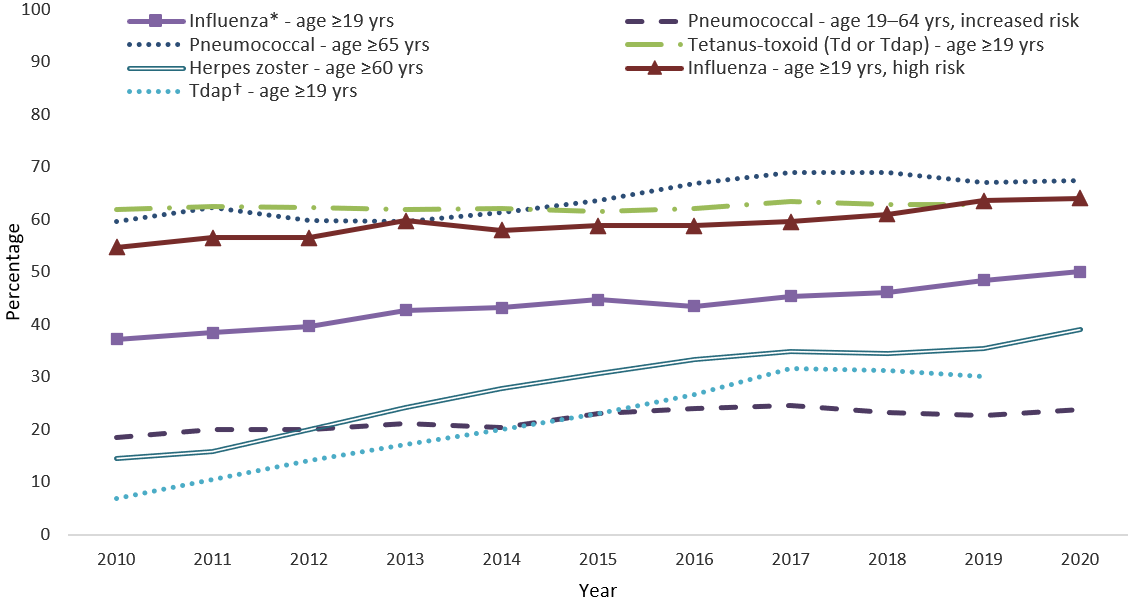
Abbreviations: Td = tetanus and diphtheria toxoids; Tdap = tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine.
* Estimates are season-specific. Year 2020 corresponds to the 2019-20 influenza season.
† Tdap vaccination coverage data among adults aged ≥65 years are available beginning in the NHIS 2012 survey.
Discussion
NHIS data from 2019 and 2020 indicate that many adults in the United States remained unprotected against vaccine-preventable diseases. Adult vaccination coverage remained similar to coverage in the year prior for most vaccines, with small increases observed for herpes zoster vaccination. Racial and ethnic differences in vaccination coverage persisted for all vaccinations, with generally lower coverage among non-White and Hispanic adults compared with White adults. Coverage for the age-appropriate composite measures was low in all age groups and in all race/ethnicity groups.
Many changes to Advisory Committee on Immunization Practices (ACIP) recommendations have occurred since 2010 for the vaccines assessed in this report. Since 1997, ACIP has recommended PPSV23 vaccination of all adults aged ≥65 years and younger adults with certain medical conditions4. In 2012, ACIP recommended PCV13 to adults aged 19–64 years at increased risk and in 2014 recommended PCV13 in series with PPSV23 for all adults aged ≥65 years; the routine recommendation for PCV13 in adults aged ≥65 years was changed in 2019 to a recommendation for administration of PCV13 based on shared clinical decision-making for adults aged ≥65 years who do not have an immunocompromising condition, cerebrospinal fluid lead, or cochlear implant, and who have not previously received PCV13567. In 2021, ACIP recommended 15-valent or 20-valent PCV for PCV-naïve adults previously eligible for pneumococcal vaccine; when PCV15 is used, it should be followed by a dose of PPSV23 ≥1 year later8. Despite many changes in recommendations, at least one dose of pneumococcal vaccine has been recommended for eligible adults throughout the period assessed in the report; since 2014, two doses of pneumococcal vaccine have been recommended for certain adults aged ≥65 years. In 2006, ACIP recommended ZVL for adults aged ≥60 years and in 2017, ACIP preferentially recommended RZV for use in immunocompetent adults aged ≥50 years over ZVL due to higher and longer-lasting efficacy910. In 2021, ACIP recommended two doses of RZV for use in immunodeficient or immunosuppressed adults aged ≥19 years11. In 2012, ACIP recommended Tdap to all adults aged ≥19 years who have not yet received a dose of Tdap, regardless of interval since the last Td shot12 and in 2019, updated its recommendations to allow either Td or Tdap to be used for the decennial Td booster, prophylaxis for wound management, and for catch-up doses13.
The largest average annual changes in vaccination coverage since 2010 have occurred for herpes zoster (2.4%) and Tdap (2.8%) vaccination while smaller increases occurred for influenza and pneumococcal vaccination. Linear trend tests from 2016 indicate increases in coverage for these vaccines have diminished in recent years compared with increases from 2010. Since the 2010–11 influenza season, ACIP has recommended annual influenza vaccination for all persons aged ≥6 months14. Though influenza vaccination coverage has continued to increase among all adults since this universal recommendation, coverage remains low with only approximately half of adults vaccinated in the 2019–20 season.
The U.S. Department of Health and Human Services has proposed a developmental HP2030 objective to assess overall adult vaccination performance. This developmental measure targets increasing the proportion of adults aged ≥19 years who receive recommended age-appropriate vaccines. This objective is a high-priority public health issue supported by evidence-based interventions; NHIS data like those presented here are a possible source of the reliable baseline data needed for it to become a core HP2030 objective.
Limitations
The estimates in this report are subject to several limitations. First, all data rely on self-report and were not validated with medical records. However, adult self-reported vaccination status has been shown to be ≥70% sensitive in one or more studies for pneumococcal, tetanus toxoid-containing, herpes zoster, and hepatitis B vaccines and ≥70% specific in one or more studies for all except tetanus and hepatitis B vaccination151617 . Second, the NHIS response rate was 59.1% in 2019 and 48.9% in 2020. Nonresponse bias can result if respondents and non-respondents differ in their vaccination rates, and if survey weighting does not fully correct for this. Third, NHIS data from 2020 at the start of the COVID-19 pandemic were obtained by telephone rather than in-person interviews and the impact of that change is unknown. While disruptions to certain vaccination services due to COVID-19 have been described1819 , vaccination coverage assessed in this report consider cumulative vaccination over time and for 2020 data, any disruptions in health care access or utilization would not be expected to show a substantial impact. Fourth, Tdap estimates are subject to considerable uncertainty and potential for bias. Respondents who reported tetanus vaccination but were unable to say whether Td or Tdap was used (19.1% of respondents reporting tetanus vaccination) were excluded from estimations of Tdap vaccination coverage. Finally, the NHIS sample excludes persons in the military and those residing in institutions, which might result in underestimation or overestimation of vaccination coverage levels.
Conclusion
Despite increases in vaccination coverage among all adults for influenza, herpes zoster and Tdap in recent years, few adults are fully vaccinated according to ACIP recommendations. Disparities in vaccination coverage by race/ethnicity were seen for all vaccines assessed. Increasing the proportion of adults who receive recommended age-appropriate vaccines and assuring equitable access to and uptake of recommended vaccines is a high-priority public health issue.
Authors
Tara C. Jatlaoui, MD, MPH; Mei-Chuan Hung, MPH, PhD; Anup Srivastav, B.V.Sc.&A.H., PhD; Peng-Jun Lu, MD, PhD; Carla L. Black, PhD; Megan C. Lindley, MPH, Ram Koppaka, MD, PhD; James A. Singleton, PhD
Immunization Services Division, National Center for Immunization and Respiratory Diseases, CDC; Leidos, Inc, Atlanta, GA
- Shen AK, Williams WW, O’Halloran AC, et al. Promoting adult immunization using population-based data for a composite measure. Am J Prev Med 2018;55:517–23.
- National Center for Health Statistics. Survey Description, National Health Interview Survey, 2020. Hyattsville, Maryland. 2021. Available at: https://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHIS/2020/srvydesc-508.pdf.
- National Center for Health Statistics. National Health Interview Survey, 2020. Public-use data file and documentation. Available at: NHIS – Data, Questionnaires and Related Documentation (cdc.gov)
- Prevention of pneumococcal disease: Recommendations of the Advisory Committee on Immunization Practice (ACIP). MMWR Recomm Rep 1997;46(No. RR-8).
- Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012;61:816–9.
- Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014;63:822–5.
- Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2019;68:1068–75.
- Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-Valent Pneumococcal Conjugate Vaccine and 20-Valent Pneumococcal Conjugate Vaccine Among U.S. Adults: Updated Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2022;71:109–17.
- Hales CM, Harpaz R, Ortega-Sanchez I, Bialek SR. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep 2014;63:729–31.
- Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep 2018;67:103–8.
- Anderson TC, Masters NB, Guo A, et al. Use of Recombinant Zoster Vaccine in Immunocompromised Adults Aged ≥19 Years: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2022;71:80-4.
- Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in adults aged 65 years and older—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2012;61:468–70.
- Havers FP, Moro PL, Hunter P, et al. Use of Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis Vaccines: Updated Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2020;69:77-83.
- Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2010;59(No. RR-8).
- Rolnick SJ, Parker ED, Nordin JD, et al. Self-report compared to electronic medical record across eight adult vaccines: do results vary by demographic factors? Vaccine 2013;31(37):3928-3935.
- Donald RM, Baken L, Nelson A, Nichol KL. Validation of self-report of influenza and pneumococcal vaccination status in elderly outpatients. Am J Prev Med 1999;16:173-177.
- Zimmerman RK, Raymund M, Janosky JE, et al. Sensitivity and specificity of patient self-report of influenza and pneumococcal polysaccharide vaccinations among elderly outpatients in diverse patient care strata. Vaccine 2003;21:1486-1491.
- Hong K, Zhou F, Tsai Y, et al. Decline in Receipt of Vaccines by Medicare Beneficiaries During the COVID-19 Pandemic — United States, 2020. MMWR Morb Mortal Wkly Rep 2021;70:245-9.
- Liow C, Payne H, Gillen M, et al. Declines in Routine Adult and Teen Vaccinations Continued in 2021. Avalere Health, January 10, 2022. Available at: Declines in Routine Adult and Teen Vaccinations Continued in 2021 | Avalere Health https://avalere.com/insights/declines-in-routine-adult-and-teen-vaccinations-continued-in-2021
